The Use of Human Fresh Tissues in the Drug Discovery Pipeline
Based in Glasgow, UK, and Maryland, USA, REPROCELL’s Center for Predictive Drug Discovery (formerly the company, Biopta) has been providing contract research services to the pharmaceutical industry since 2002. Following recent development and expansion, we are now globally recognized as the world leader in the use of fresh human tissue to better predict drug efficacy and safety during pre-clinical drug development.
Our expertise in all areas of human tissue research including sourcing, handling, and assay development, enables REPROCELL to act as your “Human Tissue Research Department”. Dedicated expert scientists are on-hand 24/7 to deliver biologically relevant data from patient derived explants (PDE) that will not only add commercial value but will also de-risk the drug development process.
One of the first companies involved in human tissue research to gain Good Laboratory Practice accreditation (2005), our quality assured data has been favorably received as part of Investigational New Drugs (IND) submissions and is increasingly being recognized by the regulators as a means to demonstrate safety or efficacy for human application.
We help our clients manage risk and save money
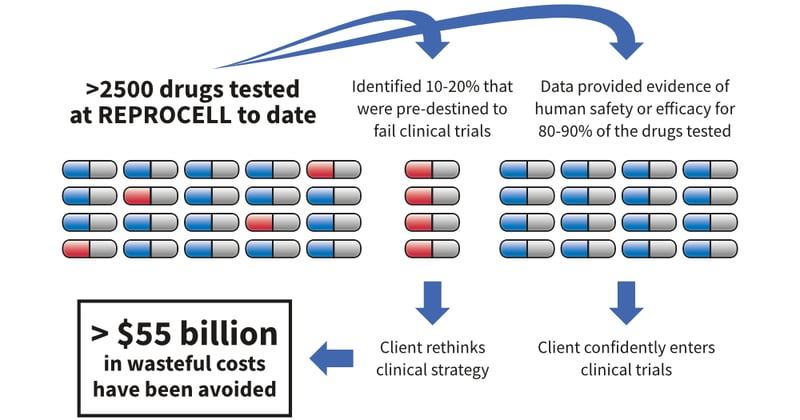
Despite record levels of investment, most drugs (80-90%) fail in clinical trials. New ways to predict which drugs will succeed are urgently required and there can be no more relevant model than fresh intact human tissues.
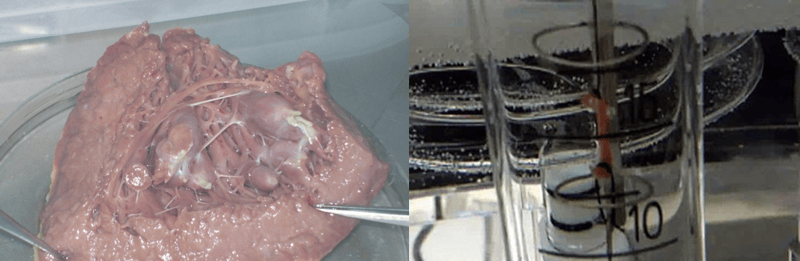
Fresh, functional human tissues are the closest possible model of how drugs will behave in patients. The above image shows human heart tissues that can be used to investigate new drugs to treat heart failure or to explore potential side-effects that may place stress on the heart. The image on the right shows an isolated strip of beating cardiac muscle to which potential new drugs can be added; the force of contraction is measured in real time and any effects of the drugs are recorded.
Demonstrating human efficacy and safety at an early stage of development has an enormous commercial value to our clients’ research programs. For 10-20% of the drugs we have tested, the client decided to rethink their clinical strategy which seemed likely to fail based on the human tissue data. We estimate that this has resulted in net savings of more than $55 billion in total costs for our clients.
REPROCELL Tissue Procurement Capabilities
Three main sources of human tissue are typically used in the drug development process:
- Tissues residual to surgery: tissues not required for diagnosis or which are generated by cosmetic procedures can be accessed rapidly and stored as fresh, fixed or frozen tissues.
- Tissues and organs from transplant procedures: organ donation rightly takes precedence over research; however, many organs cannot be used in a transplant procedure and may be consented for use in medical research.
- Clinical biopsies and biofluids, ethically obtained from specific patient groups, for example, patients with psoriasis or atopic dermatitis.
Our expert Clinical Alliance Team coordinate and monitor all aspects of the tissue procurement process, making sure that collected human tissue meets the exacting requirements of our assays.
Ethical Approval and Regulation
REPROCELL has developed an extensive tissue procurement network and is accredited as a Research Tissue Bank.
Our reputation as a leader in human tissue research is built on our ethically compliant network of human tissue partners. Ethical access to high quality tissue specimens is of the utmost importance to REPROCELL and we have developed a robust network of trusted clinical collaborators and tissue banks, in both the UK and USA, from which we can receive human tissue with total confidence.
We work in accordance with all relevant legislation and comply with the highest standards of ethics and quality control. REPROCELL has ethical approval for its research projects and deals with all ethical and legal requirements including full informed consent of every patient donating tissue, to allow your research project to commence swiftly, secure in the knowledge that all appropriate consents and approvals are in place.
Download our Bioethics Policy (PDF)
Obtaining human data on your drug compounds
Delivering fresh human data for your test compound couldn’t be easier:
- Contact our experts to discuss a protocol designed specifically for your needs
- We will send you a fully-costed proposal and timeline
- Send the test compound (drug, cosmetic or chemical) to REPROCELL
- REPROCELL will provide you with raw data and a written report
- Tissues and test compounds can be returned to you for further testing




![Wire myography: the ultimate guide [protocol included]](https://www.reprocell.com/hs-fs/hubfs/REPROCELL-04.06.18_0163.jpg?width=756&height=425&name=REPROCELL-04.06.18_0163.jpg)