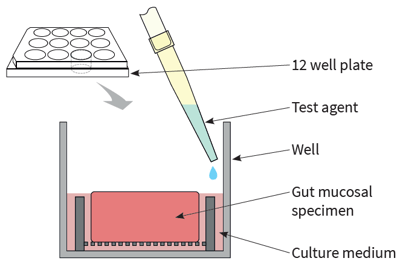
Anti-inflammatory drug effects in IBD tissue
The incidence of IBD is increasing and there is a clinical need for safer, more precise IBD treatments. However, failure to predict the safety and efficacy of drugs during preclinical research means that attrition rates are high. REPROCELL offers the most clinically relevant IBD assay on the market, which uses human tissue donated by real IBD patients. Our living tissue model captures the heterogeneous landscape of IBD by combining drug efficacy data with a medical history of each human donor.
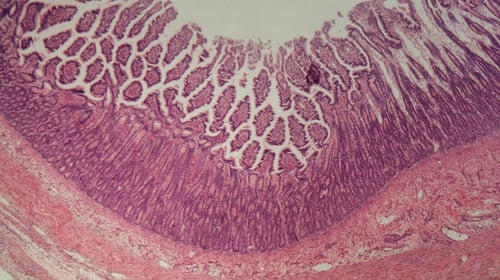
Microscope histological image of IBD colitis intestine
Meet clinical needs in IBD research
Using our IBD intestinal explant assay, we can investigate the anti-inflammatory effects of your test drugs to treat Crohn's disease or ulcerative colitis. As inflammation is a defining feature of IBD, the tissue resections (ileum or colon) are received residual to surgery with inflamed areas of the intestine determined by a pathologist.
All tissues are rapidly transported to REPROCELL as fresh living tissues 24/7 for processing at our laboratories in the USA or UK. Similar experiments can be conducted in non-human preclinical models of IBD to allow cross-species comparisons. The resultant organocultures can be exposed to a range of test agents for up to 24 hours.
“Biopta’s human tissue services have played a critical part in our compound selection and have added considerable value to our lead compound”
— President & Head of R&D, Canadian Biotech
Access intestinal tissue from IBD donors
More scientists would like to use human tissue in their research. However, poor biobanking infrastructure and technical difficulties present significant barriers. Through our global network of biorepositories, we can access a frequent supply of high-quality intestinal tissues from Crohn’s and Ulcerative Colitis (UC) patients. They are then maintained for 24-hours in our ex vivo culture system, where they can be exposed to a variety of novel and standard-of-care drugs.
Why use human tissues instead of traditional animal models?
- Avoid species differences
- Increase therapeutic relevance
- Improve understanding of IBD pathology
- Proof of concept in diseased human tissues
- Reduce late-stage failures
- Strengthen IND submissions
- Explore complex cell interactions in whole tissue
Five reasons to choose our Human IBD Fresh Tissue Assay:
- Access the only commercially available assay using intact, living human tissues.
- Add commercial value by demonstrating efficacy in tissues from your target patient group.
- Identify lead test agents with likelihood of success in patients.
- Compare your test agents to current standard of care drugs.
- Combine with REPROCELL’s molecular biology services to relate phenotype to genotype.
Relate drug response to clinical history
Precision medicine aims to deliver the right drug to the right patient at the right dose. However, most presently available drugs fail to work in a high percentage of patients; including treatments for IBD.3 At REPROCELL, we offer early-stage characterization of the patient population. By combining measurements of drug efficacy with clinical histories and access to state-of-the-art molecular phenotyping, we can investigate inter-individual variation in drug response at the preclinical stage.
Functional validation of healthy and diseased tissues
As part of our functional validation, biopsies are screened for inflammatory mediators commonly upregulated in IBD.
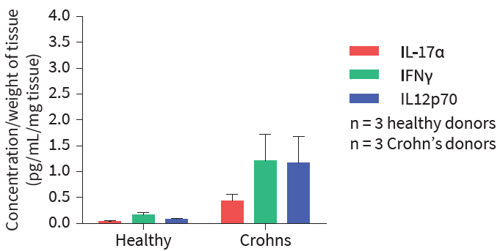
Figure 1: In this example, over 40 inflammatory mediators were measured to investigate the cytokine release profiles in healthy and diseased tissue. The disease group showed greatly increased levels of a range of key cytokines and chemokines commonly upregulated in IBD, including IL-17α, IFNγ and IL-12p70.
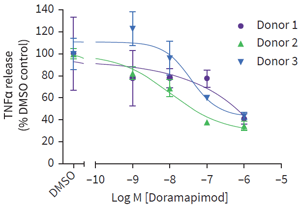
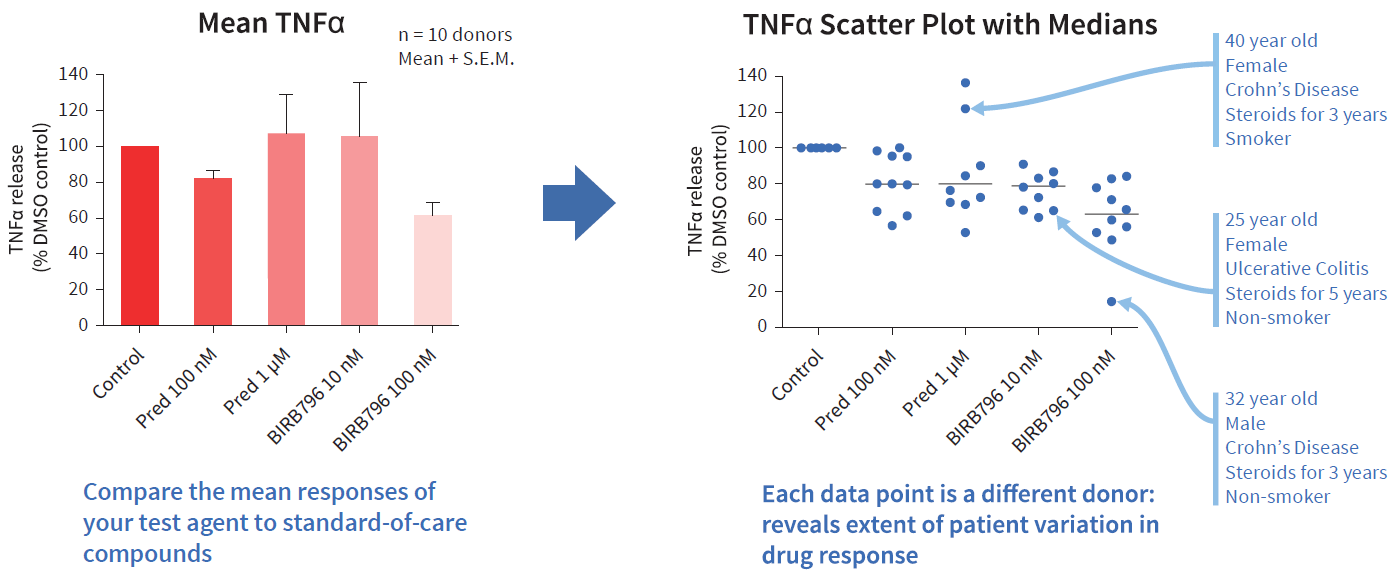
Figure 2: Intestinal biopsies were obtained from 3 UC patients. Following an 18-hour culture period, TNFα release was measured across a wide concentration range of the test agent Doramapimod (see graph). Differences in response were evident across patient samples, suggesting variation in the UC patient population, as is known to occur clinically.
What is included in our IBD drug discovery services?
- A customized project to meet your research goals.
- An expert Study Director assigned to manage each project as your single point of contact from initiation to report.
- Rapid access to human healthy and diseased tissues, through our industry-leading human tissue
network. - GLP projects available.
- Ownership of all data generated during the project.
This assay uses gut biopsies from IBD donors to assess the anti-inflammatory effects of your test articles.
Other assays can be found in our drug discovery assay catalog.
References
- Ananthakrishnan. Epidemiology and risk factors for IBD. Nature reviews Gastroenterology and Hepatology. 2015, 12:205-17.
- Porter et al. Can we target endogenous anti-inflammatory responses as a therapeutic strategy for inflammatory bowel disease? Inflammatory Bowel Disease. 2018, 00:1-11
- Schork. Personalized Medicine: Time for one-person trials. Nature. 2015, 520:609-611.
- Bowes et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nature Reviews Drug Discovery. 2012, 11:909–22.
- Denson et al. Challenges in IBD Research: Update on progress and prioritization of the CCFA’s Research Agenda. Inflammatory Bowel Disease. 2013, 19:677-682.
- Jackson et al. The use of human tissue in safety assessment. Journal of Pharmacological and Toxicological Methods (2018).
- Edwards et al. Human tissue models for a human disease: what are the barriers? Thorax. 2014, 0:1-3.