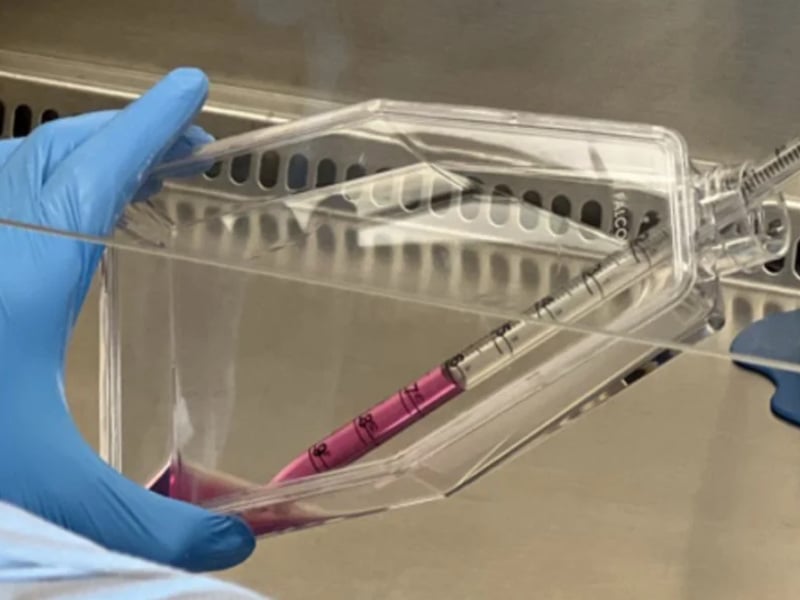
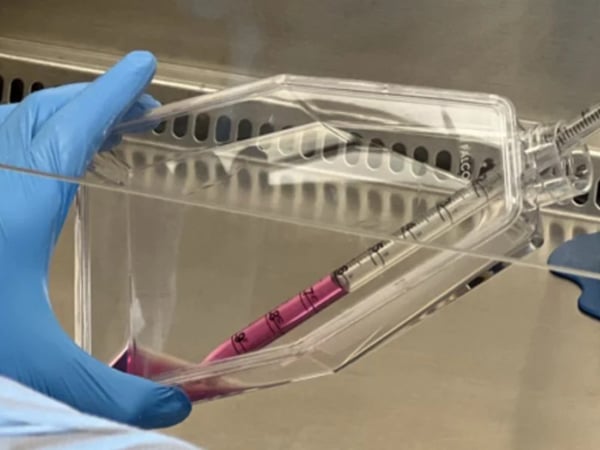
Clinical Stem Cell Services
Our experts in cell therapy manufacturing will take you through all the stages of your regenerative medicine project.
Developing cellular therapies requires strict adherence to manufacturing and regulatory standards that are different for each national regulatory agency. REPROCELL can help you through all stages of your regenerative medicine project.

Cell-based therapies have significant potential to provide patient relief in a broad range of disease areas, from cancer to degenerative disorders. Many of these projects start with iPSCs (induced pluripotent stem cells) or with MSCs (mesenchymal stem cells). Master cell banks (MCBs) can be effectively generated from these stem cells, ensuring product consistency while managing risk by eliminating contamination and genetic instability.
Specifically, iPSCs are straightforward to manipulate genetically, and the modified iPSCs open the path for innovative therapeutics. Some examples include gene correction for the therapeutic rescue of genetic diseases, the addition of genes at precise genome sites, and the creation of hypoimmune lines that can overcome HLA mismatches.

Whether your project starts with iPSCs or MSCs as the source for your stem cell-based therapeutic project, or if you are interested in clinical gene editing, REPROCELL can assist you!
REPROCELL is a global company that serves customers all around the world. Our worldwide presence ensures that our client's cell therapy projects are compliant with the regulatory standards and guidelines of the FDA, EMA, and PMDA. We will provide the necessary quality and regulatory documents including donor eligibility, CoAs, batch records, traceability documentation, and quality technical agreements for your GMP Master Cell Bank or Cell Therapy product.
REPROCELL’s Clinical Stem Cell Services
More about REPROCELL’s Clinical iPSCs and MSCs
Reviews
Review article (offsite)
How to find the right end-to-end developer for manufacturing your cGMP-compatible iPSCs
From β-islets for diabetes to dopaminergic neurons for Parkinson’s disease, induced pluripotent stem cell (iPSC)-derived, haplotyped, and multiplex-engineered allogeneic cell therapeutics empower the development of effective and safe Cell and Gene Therapy Products (CGTs) that could be manufactured on a large scale in a cost-effective manner and provide a functional cure for diseases that plague humankind today.
Bioinformant
Review article (offsite)
Making iPSC-derived therapies a clinical reality
Since their invention in 2006, induced pluripotent stem cells (iPSCs) have revolutionized how researchers approach disease modelling, drug discovery research, and drug development. Derived from adult somatic stem cells rather than embryos, iPSCs have allowed scientists to study human biology with increased depth, reproducibility, scalability, and translatability. This is especially true for diseases where animal models lack clinical relevance, and where primary tissues are difficult to access.
Stem Cell Research and Technology
Webinars
Webinar: Things to consider when searching for a supplier of cGMP iPSCs
Dr Mahendra Rao
Webinar: Reproducible differentiation of pure ovarian support cells from clinical-grade hiPSCs as a novel infertility treatment
Dr Bruna Paulsen, PhD
VP of Manufacturing and Therapeutic Development, Gameto
Other information
Are you looking for something else?
In addition to our clinical GMP iPSC production and GMP MSC production services, we have a range of other stem cell services and products.
Find out more about REPROCELL’s Clinical Stem Cell Services
If you are interested in finding out more, please make an inquiry using the form below. Our stem cell experts will be happy to arrange a free consultation to discuss your project in more detail.
Contact our experts
Discover more
Resources
- FAQ: Clinical iPSCs
- FAQ: Clinical MSCs
- FAQ: iPSC-Derived Exosomes
- Making iPSC-Derived Therapeutics a Clinical Reality – our external article in the European Biopharmaceutical Review.
Gene Editing Services
Latest in Stem Cells

iPSC-, MSC- and iMSC-Derived Exosome Therapeutics: The Cell-Free Future of Regenerative Medicine
Explore the future of regenerative medicine with iPSC-, MSC-, and iMSC-derived exosome therapeutics, focusing on their advantages, challenges, and clinical potential.
19 November 2025

Current Landscape of FDA Stem Cell Approvals and Trials 2023-2025
Discover the latest advancements and FDA approvals in stem cell therapies, including iPSC and MSC treatments, reshaping the 2023-2025 clinical landscape.
02 September 2025

Why Early Genetic Variant Detection in iPSC Cultures Is Critical for Safe and Regulatory-Compliant Cell Therapies
Explore the crucial role of early genetic variant detection in iPSC cultures for ensuring safe, effective, and regulatory-compliant cell therapies. Learn about risk profiling and REPROCELL’s solutions.
01 August 2025











%20cells-1.png?width=800&name=ReproMSC3%20(RCRP025)%20cells-1.png)