The Power of mRNA Reprogramming
The emergence of mRNA reprogramming offers a precise, efficient, and xeno-free approach to generating induced pluripotent stem cells (iPSCs). These versatile cells hold immense potential for personalized medicine, disease modelling, and drug discovery. REPROCELL has led the way in mRNA reprogramming, in 2010 becoming the first company to commercialize mRNA-based reprogramming technologies. REPROCELL’s proprietary StemRNA™ Reprogramming Technologies for clinical and research applications are at the forefront of this technological evolution, enabling scientists to unlock new frontiers in cellular therapy.
What is mRNA Reprogramming, and Why Is It Superior?
Reprogramming methods, such as retroviruses, lentiviruses, and transposons, risk genomic integration, leading to potential mutational instability and tumorigenesis. Non-integrative alternatives like episomal vectors, sendai virus, adenoviruses, and proteins are safer, but either lack efficiency or the vectors are retained in the iPSCs.
In contrast, mRNA reprogramming stands out for its unmatched combination of high efficiency and superior safety. It utilizes mRNAs to introduce a specifc set of reprogramming factors into somatic cells, convert them into iPSCs. A critical safety concern for clinical applications is the risk of genomic integration and residual reprogramming vectors in the cell. Unlike other methods, the mRNA approach avoids genomic integration due to the natural cellular process of degrading foreign RNAs. Additionally, mRNA reprogramming relies on transient signals, which minimize mutation risks and preserve the genetic integrity of iPSCs (Bailly et al., 2022).
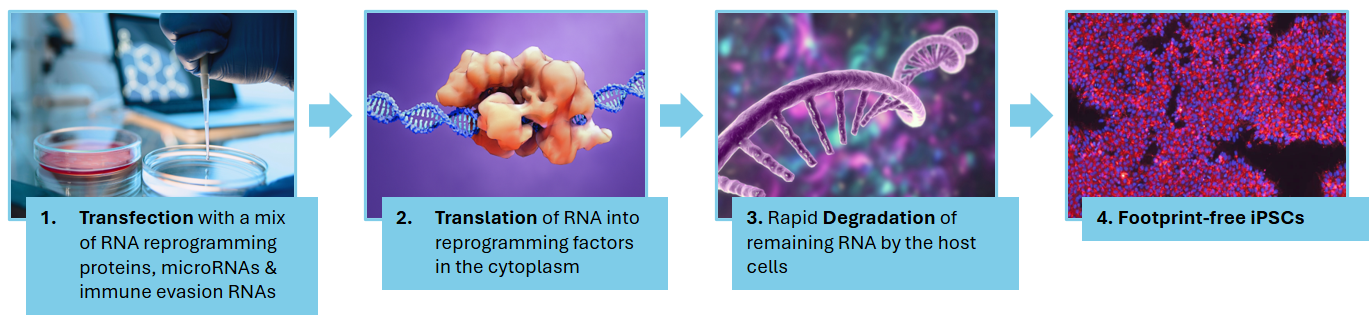
Key Advantages of mRNA Reprogramming:
- High Efficiency: Delivers consistent results with rapid colony formation.
- Safety: Non-integrative, meeting stringent standards for clinical applications.
- Scalability: Ideal for both research and industrial-scale clinical projects.
Discover how REPROCELL’s innovative StemRNA Clinical Reprogramming Technology can help with your cell therapy project.
REPROCELL’s (Stemgent®) StemRNA™ 3rd Gen Reprogramming Kit: Advanced Tool for Research-grade iPSC Generation
At the heart of REPROCELL’s offering is the 3rd Gen Reprogramming Kit—a cutting-edge solution designed to streamline the generation of iPSCs for research purposes under xeno-free conditions. Leveraging the power of proprietary non-modified RNAs (NM-RNAs), this kit ensures unparalleled reprogramming efficiency and compatibility with a variety of workflows.

Figure 2. REPROCELL’s (Stemgent®) StemRNA™ 3rd Gen Reprogramming Kit (00-0076)
Highlights of the Kit
- Optimized Reprogramming Factors: Combines OSKMNL (Oct4, Sox2, Klf4, c-Myc, Nanog, Lin28) with immune evasion factors (EKB) to enhance efficiency while mitigating immune responses. Double-stranded microRNAs enhance reprogramming efficiency, providing iPSC clones with high efficiency (up to 4% from fibroblasts, up to 3% from EPCs, up to 0.5% from UPCs).
- Flexible Protocols: Compatible with substrates like Corning® Matrigel® and iMatrix-511 for customizable workflows.
- Enhanced Performance in Hypoxia: Hypoxic conditions (5% O₂) boost colony yields, making it ideal for advanced lab setups.
- Scalability: Designed for use in multi-well formats, facilitating simultaneous reprogramming of multiple samples.
Reprogramming Timeline
Day 0: Plate fibroblasts on iMatrix-511-coated wells.
Day 1-4: Perform daily RNA transfections in NutriStem medium.
Day 5-14: Maintain cultures with daily medium changes.
Day 10-14: Identify and pick iPSC colonies for further expansion.

Figure 3. Timeline of Fibroblast Reprogramming into iPSCs using StemRNA™ 3rd Gen Reprogramming Kit
Protocol for Stemgent® StemRNA™ 3rd Gen Reprogramming Kit
This easy-to-follow guide outlines a streamlined method for reprogramming adult and neonatal human fibroblasts into iPSCs using the Stemgent® StemRNA™ 3rd Gen Reprogramming Kit. The protocol ensures a xeno-free culture environment using non-modified RNAs (NM-RNAs) and is optimized for use with iMatrix-511 and NutriStem® hPSC XF medium.
Protocol: Stemgent® StemRNA™ 3rd Gen Reprogramming Kit for Reprogramming Adult and Neonatal Human Fibroblasts

Figure 4. Clinical-grade iPSC production
Clinical-Grade iPSCs by REPROCELL: mRNA Reprogramming Services & off-the-shelf lines
For researchers and clinicians aiming to translate their findings into therapies, our proprietary StemRNA™ Clinical Reprogramming Technology provides an unparalleled solution. Our approach ensures the production of high-quality, clinical-grade iPSCs and aligns with regulatory standards from the key agencies FDA, EMA, and PMDA.
Why Choose REPROCELL’s Services for clinical-grade iPSCs?
- Clinical-Grade Production: Adheres to strict guidelines, ensuring cells are suitable for therapeutic applications.
- Customizable Solutions: Offers exclusive reprogramming services, including patient-specific iPSCs for personalized medicine.
- Rapid Turnaround: Combines advanced technology and streamlined processes for efficient project completion.
- Global Expertise: Supported by a diverse, global team of stem cell scientists and a proven track record in regenerative medicine.
- License: At REPROCELL, we streamline your iPSC projects by offering clinical and commercial licenses, positioning ourselves as your hassle-free, one-stop solution for all your needs.
Whether you are conducting preclinical research or developing cellular therapies, REPROCELL’s comprehensive service platform provides the reliability and precision needed to advance your projects.
Conclusion
mRNA reprogramming represents a paradigm shift in iPSC technology, offering unmatched efficiency, safety, and scalability. With REPROCELL’s 3rd Gen Reprogramming Kit for research-grade iPSC generation and specialized mRNA reprogramming services for clinical-grade iPSCs, researchers and clinicians can accelerate their journey from discovery to therapy.References
- Bailly, A., Milhavet, O. and Lemaitre, J.M. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics.14(2):317. 2022



.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)




