- Clinical Stem Cell Services
- GMP iMSC & MSC Production Service
GMP‑Grade iMSC & MSC
Manufacturing & MCB Generation
REPROCELL delivers GMP Master Cell Banks (MCBs) of mesenchymal stem cells (MSCs)—also known as Mesenchymal Stromal Cells and Medicinal Signaling Cells—manufacturing services for conventional MSCs and iPSC-derived MSCs (iMSCs), tailored to your next clinical project. Our production adheres to US FDA, European EMA, and Japanese PMDA standards to ensure consistent, therapeutic-grade quality. Off-the-shelf GMP iMSC and MSC banks are also available for immediate clinical use—streamlining your MSC therapy supply chain.
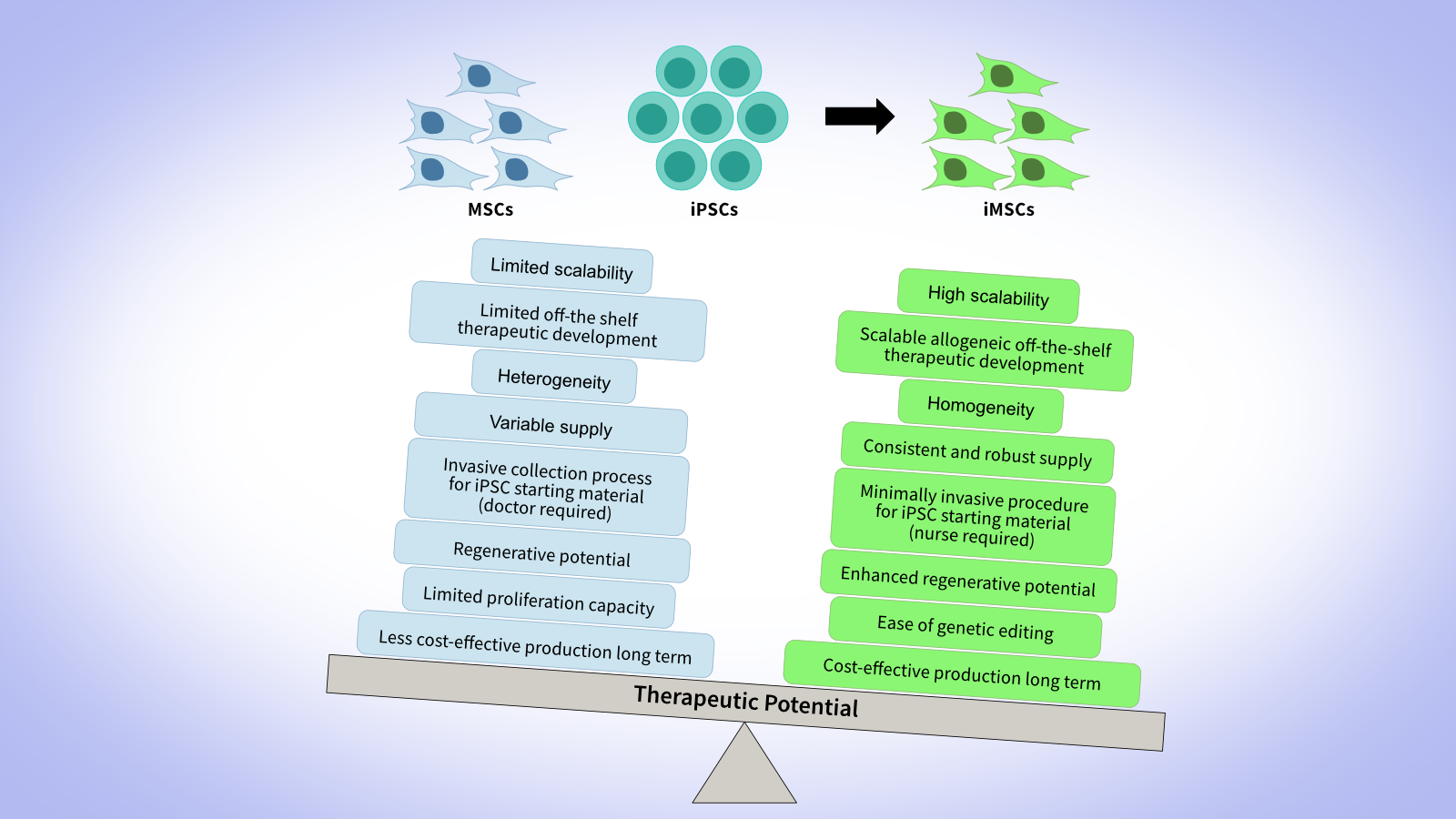
Why choose GMP-grade MSCs?
MSCs, also known as Mesenchymal Stromal Cells or Medicinal Signaling Cells, have received a lot of interest, both as direct therapeutic agents and for their potential to differentiate into clinically relevant somatic cell types and tissues. More than 1000 clinical trials using MSCs in a wide variety of therapeutic areas have been reported.
Why choose GMP-grade iMSCs?
Derived from iPSCs, our GMP-grade iMSCs and research-grade iMSCs offer a scalable, consistent alternative to primary MSCs. They deliver higher batch‑to‑batch uniformity and potency, enabling for a large-scale, robust, cost-effective MCB production.
Discover why iMSCs are capturing the spotlight—and how they stack up against traditional primary MSCs—on our latest blog:

Primary MSC Isolation and GMP‑Grade Expansion
Conventional primary MSCs can be isolated from multiple sources—such as bone marrow, adipose tissue, cord blood, or placenta—purified and robustly expanded under GMP‑grade conditions to yield therapeutic‑scale cell quantities. Alternatively, iMSCs provide a scalable, xeno‑free source of MSCs. Both primary MSCs and iMSCs support scalable expansion and directed differentiation into cartilage, muscle, bone, and other clinically relevant tissue types, positioning them as versatile platforms in GMP MSC production and cell therapy development.
Scalable GMP MSC/iMSC MCB Production and CDMO Partnerships
At our REPROCELL USA facility, we specialize in producing custom GMP-grade iMSCs and iMSC MCBs—either derived from our StemRNA Clinical iPSC clones or your company’s proprietary iPSC line.
Whether you are initiating a new preclinical study or preparing for late-stage development, our dedicated GMP manufacturing facility is equipped to produce clinically compliant iMSC MCBs at scale. With our European partner, we provide cGMP‑manufactured MSCs, MCBs, and Working Cell Banks (WCBs) covering custom and off-the-shelf services for clinical and research applications worldwide.
GMP MCB Manufacturing Service at
REPROCELL USA
We now offer full-service GMP-grade MCB production for iPSCs and iMSCs at our cutting-edge Beltsville, MD facility:
- A closed, modular Cytocentric Xvivo system
- Class 100 (ISO 5) containment
- Complete with HEPA‑filtered,
- Monitored environments
This modular system drastically reduces contamination risk while accelerating turnaround times.



GMP MCB Manufacturing Service at
Histocell in Europe
We have partnered with Histocell, a Spain‑based CDMO with a fully authorized GMP‑ATMP facility in Bilbao for both clinical iPSC and MSC therapies:
- 13+ years of GMP experience
- Seven clean rooms
- Automated fill/finish
- Multiple QC labs
- Ability to run autologous and allogeneic batches at both pilot and clinical scale
We support everything from custom MSCs and iPSC MCB manufacturing to secretome/exosome products.



Our MSC and iMSC-related services:
Isolation, Expansion & Differentiation
- Isolation and expansion of autologous or allogeneic adult adipose-derived MSCs
- Expansion of autologous and allogeneic adult bone marrow MSCs
- Mesodermal Trilineage differentiation of MSCs (chondrocytes, osteocytes, myocytes)
- Production and characterization of cell-derived products like secretome and exosomes
We provide all regulatory QC specified by the EMA or FDA, and all the required documentary support for the GMP product.
FAQs
We Can Also Help with Your Research MSC Project
R&D lab – GMP-like production and QC
- Fully equipped Molecular biology lab includes:
- Cytofluorometry
- Spectrophotometry
- Immunohistochemistry
- Suitable for general R&D projects
- Provides a GMP-like tech-transfer phase
- Uses Quality Control similar to cell therapy drugs

Off-the-Shelf GMP MSCs and Exosomes from Cellcolabs
(Solna, Sweden)

20+ Years of Karolinska Research Supports Cellcolabs MSC Production
Discover our GMP-grade MSCs—our "Ready-to-Use GMP MSCs" are produced in collaboration with Cellcolabs (Solna, Sweden), drawing on over 20 years of Karolinska Institute research and clinical trial experience. These young, extensively screened donor-derived bone marrow MSCs are expanded in xeno-free media, cryopreserved at passage 3, and thoroughly QC-tested for sterility, karyotype, viability, and surface marker expression, ensuring a reliable, off-the-shelf asset for regenerative and immunomodulatory therapy projects.
Ask our experts about our GMP MSC production service
Discover more
Resources
- FAQ: Clinical iPSCs
- FAQ: Clinical MSCs
- FAQ: iPSC-Derived Exosomes
- Making iPSC-Derived Therapeutics a Clinical Reality – our external article in the European Biopharmaceutical Review.
Gene Editing Services