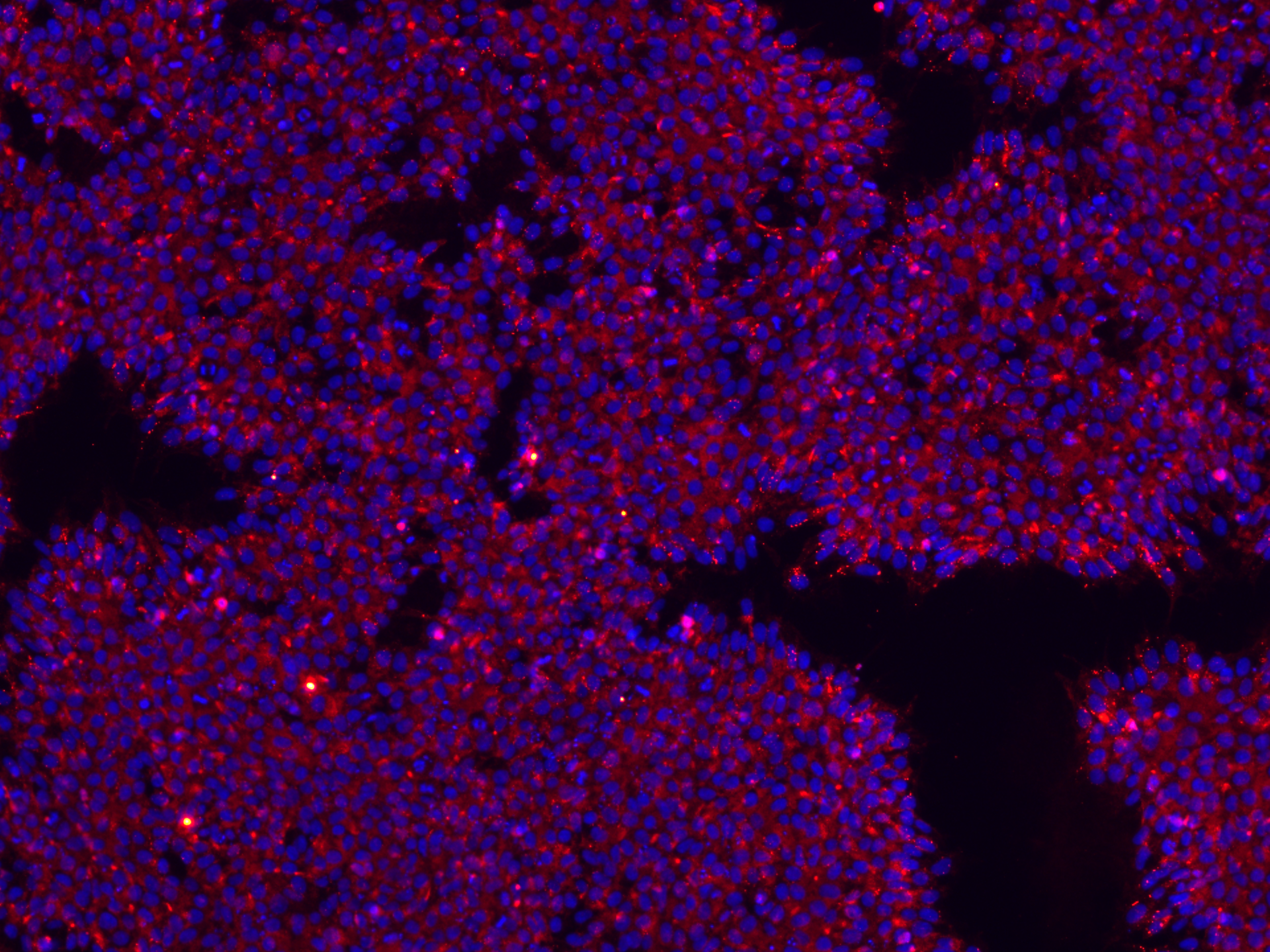
- Clinical Stem Cell Services
- Clinical Grade iPSC Production Service
StemRNA™ Clinical Grade iPSCs
for Your Cell Therapy Application
REPROCELL can help with iPSC Manufacturing Services for your cell therapy product.
Why choose our clinical-grade iPSCs for your iPSC-derived cell therapies?
REPROCELL is a trusted partner for cell therapy developers who are seeking robust, high-quality, clinical-grade induced pluripotent stem cell (iPSC) lines that meet industry standards.
As we have global access to a roster of donors, we can procure the starting material for your cell therapy project, perform the mandatory viral and donor profile screenings, and ensure that donor samples are fully consented for clinical and commercial use. The informed consent forms contain specific information fundamental to iPSC-related cell therapy products. Our experts use our proprietary footprint-free RNA reprogramming technology and clinical-grade media reagents to generate StemRNA™ Clinical iPSC Seed Clone Lines.
Clinical iPSC based Cell Therapy Service – Overview

REPROCELL’s StemRNA™ Clinical Seed iPSCs serve as a reliable foundation for various cell therapy programs by ensuring safety, consistency, and regulatory compliance. These iPSC Seed Clones can be expanded in a GMP environment to manufacture Master Cell Banks (MCB), which can be further downstream processed to generate a final drug product.
Our clinical-grade iPSCs are currently being evaluated by many biopharma companies globally, have been included in regulatory filings, and are IND approved.
REPROCELL offers GMP MCBs of iPSC, iMSC, and MSC manufactured by our stem cell experts in compliance with the regulatory standards and guidelines of the US FDA, European EMA, and Japanese PMDA. Our regulatory support team will provide all necessary quality and regulatory documents, such as donor eligibility, CoA, batch records, traceability documentation, and quality technical agreement for your cell therapy project filing with the selected agency.
Every StemRNA Clinical Grade iPSC seed clone, its corresponding donor material, and any subsequent GMP MCB batch are assessed for genetic integrity by performing a combined approach of two complementary techniques:
We conduct low-resolution G-band karyotyping to ensure a normal broad structural state of the chromosomes, including the absence of any numeric aberrations and structural variants. For higher resolution analysis, we perform NGS-based oncogenic analysis to profile for genetic variants in over 400 cancer-related genes for a deeper molecular insight.

For each ready-to-use clinical iPSC clone, we provide an oncogenic report that analyses for high-impact genetic variants and interprets them using publicly available clinical databases.
FDA Drug Master File (DMF)—exclusively covering iPSCs manufactured at our U.S. site and accessible via a simple Letter of Authorization (LOA).
The StemRNA™ Clinical Process
REPROCELL's clinical process starts with collecting skin for fibroblast isolation from donors who have provided informed consent for clinical and commercial use and have been thoroughly screened. We then reprogram the fibroblasts to iPSCs using our proprietary StemRNA™ Clinical Reprogramming Technology. Multiple iPSC clones are isolated and quality controlled to create StemRNA Clinical iPSC Seed Clones, which are suitable for further expansion into a GMP iPSC MCB. The Seed Clones can also be used directly for StemEdit™ clinical gene editing before expansion to the MCB.
These clones can be accessed in three ways:
- Exclusive StemRNA Clinical Seed Clones: We can generate them for you, starting with a donor that matches your criteria, or you can supply the cryopreserved clinical fibroblasts.
- Ready-to-use StemRNA Clinical iPSC Seed Clones: We have a bank of clones available for your evaluation.
- StemRNA Clinical iPSC Pilot Clones: We created corresponding research-use clones from the Clinical Seed Clones.
FAQs
The difference between Seed Clones and Pilot Clones
StemRNA Clinical iPSC Seed Clones (Clinical-use)
These cells are suitable for clinical use through subsequent regulated and approved processes, including activities resulting in a GMP Master Cell Bank and Working Cell Bank. StemRNA Clinical iPSC Seed Clones are generated on the principles of GMP and covered by a rigorous quality control (QC) process that is compliant with US FDA, European EMA, and Japanese PMDA regulations.
StemRNA Clinical iPSC Pilot Clones (Research-use)
These cells are intended for evaluation purposes only, and they are NOT suitable for downstream therapeutic applications. The ready-to-use StemRNA Clinical iPSC Pilot Clones are created in a research setting and expanded from our GMP-like StemRNA Clinical iPSC Seed Clones. These Pilot clones offer a more cost-effective way to explore the potential of our Clinical Seed iPSCs for developing and optimizing your processes.
Both the Seed Clones and the Pilot Clones include an evaluation period to determine their suitability for your process. During the evaluation period, sufficient vials of the Seed Clones will be reserved for you to carry forth into GMP iPSC Master Cell Bank generation after successful evaluation.
Example of Available StemRNA Clinical iPSC Lines
|
Donor |
Gender |
Age |
Race |
Clinical Status |
Blood Type |
|
Donor A |
Female |
25 |
Asian |
Healthy |
B+ |
|
Donor B |
Female |
23 |
Caucasian |
Healthy |
A+ |
|
Donor C |
Female |
22 |
Caucasian |
Healthy |
O+ |
|
Donor D |
Male |
23 |
Caucasian |
Healthy |
A+ |
|
Donor E |
Male |
27 |
Caucasian |
Healthy |
A+ |
|
Donor F |
Male |
60 |
Caucasian |
Healthy |
O− |
- HLA and KIR (Killer-cell immunoglobulin-like receptors) genotyping data are available for all donors.
- Multiple clones are generally available from each donor.
- Different clonal lines are validated for their differentiation potential into various specific cell types. Depending on the iPSC clone, this includes: HSCs (hematopoietic stem cells), NSCs (neural stem cells), NK (Natural Killer) cells, astrocytes, and iGRPs (induced glia-restricted progenitors) and others.
- If you have specific donor criteria, we can identify and screen a donor that meets your needs.
- New differentiation data on the various lines are regularly becoming available, which may make it easier to pick the right clonal line to test in your therapeutic project.
If you want to find out more about our clinical stem cell services, please contact our experts.
News and Advantages of REPROCELL's Clinical RNA iPSCs
REPROCELL is a pioneer in using mRNA for generating iPSCs. mRNA generates high-quality iPSCs that are uniquely suited for clinical applications.
- mRNA is not retained in cells, eliminating concerns of retention of reprogramming vectors.
- mRNA cannot be integrated into the genome.
- iPSCs reprogrammed with mRNA show lower rates of genomic abnormalities
REPROCELL’s StemRNA™ Clinical iPSC Seed Stock Clones enabled a historic breakthrough in assisted reproduction: Gameto’s Fertilo platform delivered the world’s first live birth involving ovarian support cells entirely manufactured from our clinical-grade iPSCs.
These ovarian support cells closely mirror the natural ovarian environment, allowing in vitro oocyte maturation with minimal hormonal intervention, reducing patient burden and potential complications such as ovarian hyperstimulation syndrome.
An IND officially was approved by the U.S. Food and Drug Administration, allowing launch of the first-ever Phase 3 iPSC-based therapy trial in the United States, marking the first application of human iPSC-derived cells in IVF and redefining what cell-based fertility therapies can achieve.
More information
Reference
Bruna Paulsen et al. Development of human induced pluripotent stem cell-derived ovarian support cells as a clinical-grade product for in vitro fertilization. Cell Stem Cell, 33, 1-15 (5 Feb 2026).
DOI: 10.1016/j.stem.2025.12.020
QC data of typical StemRNA Clinical iPSCs Clones
Morphology, P6+3

Karyology

Flow cytometry analysis

3-germ layers differentiation potential measured by RT qPCR

Typical quality control assays of Clinical StemRNA iPSC Lines. To verify the suitability of the StemRNA iPSCs as a starting material for clinical programs, each clonal line is validated in several assays, including colony morphology and growth rate, karyotyping, pluripotency marker expression by flow cytometry, and directed differentiation to measure their functional pluripotency potential. Only the clones that pass all specifications of batch release assays are suitable for advancement to scale-up for GMP Master Cell Bank manufacturing.
Neural differentiation capability of clonal iPSC line from donor B
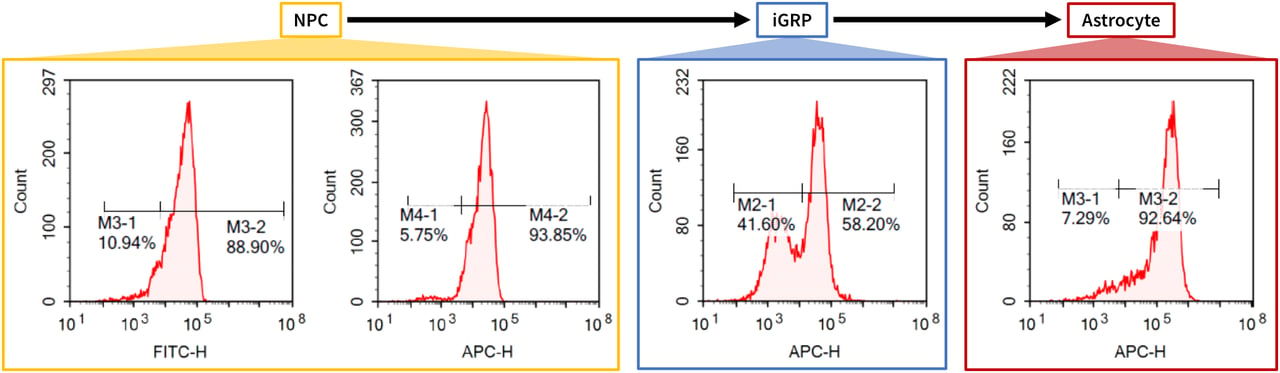
Targeted Differentiation Capacity into Neural Lineage of a Typical Clone. Clinical iPSCs were sequentially differentiated into cells of the neural lineage and characterized by fluorescence cytometry. These results show that at various stages of differentiation, the cells express markers typical for Neural Progenitor Cells, iGRPs (induced glia restricted progenitor cells), and astrocytes.
(Data acquired in-house.)
Natural Killer (NK) cell differentiation capability of clinical iPSCs

Expression of key NK cell surface markers on day 36 of differentiation induction from clinical iPSCs. REPROCELL's clinical iPSCs were differentiated into NK cells (iNK cells) and characterized by flow cytometry. CD45+CD56+ NK cells also expressed NKp44 (CD336), NKp46 (CD335), and NKG2D (CD314). These results demonstrate that NK cells derived from our clinical iPSCs express markers typical of mature and functional NK cells.
Data acquired in collaboration with Tokyo Metropolitan Institute of Medical Science in Japan.
Live imaging of human iNK cells engulfing tumor K562 cells. The elongated human iNK cells demonstrated cytotoxic properties when co-cultured with K562 cells (a human chronic myeloid leukemia cell line), labeled with the cell staining reagent CFSE (green round cells). These results show that iNK cells exhibit mature function in identifying and destroying foreign cells.
Data acquired in collaboration with Tokyo Metropolitan Institute of Medical Science in Japan.
You can also outsource your clinical or research gene editing project to REPROCELL by providing your own iPSC line or using our StemRNA iPSC Clones.
Commercial license available
Our clients often have questions about donor consent, but all our tissue donors have fully consented to clinical and commercial use of their cells.
We can also provide the necessary clinical and commercial licenses for your project – making us a hassle-free one-stop solution provider for your iPSC needs.
Contact our experts
At REPROCELL, our scientists understand that your custom iPSC project must be as unique as your research. If you have any questions about how our Clinical iPSC Generation Service can help you advance your project, please make an inquiry using the form below.
Discover more
Resources
- FAQ: Clinical iPSCs
- FAQ: Clinical MSCs
- FAQ: iPSC-Derived Exosomes
- Making iPSC-Derived Therapeutics a Clinical Reality – our external article in the European Biopharmaceutical Review.
Gene Editing Services
On the REPROCELL blog
Latest in Stem Cells

From StemRNA™ Clinical Seed Clone to GMP-Ready Cell Therapy Programs
Discover how REPROCELL’s StemRNA™ Clinical iPSC Seed Clones streamline the path to GMP-ready cell therapy, simplifying regulatory processes and enabling scalable, high-quality manufacturing.
27 February 2026

iPSC-, MSC- and iMSC-Derived Exosome Therapeutics: The Cell-Free Future of Regenerative Medicine
Explore the future of regenerative medicine with iPSC-, MSC-, and iMSC-derived exosome therapeutics, focusing on their advantages, challenges, and clinical potential.
19 November 2025

Current Landscape of FDA Stem Cell Approvals and Trials 2023-2025
Discover the latest advancements and FDA approvals in stem cell therapies, including iPSC and MSC treatments, reshaping the 2023-2025 clinical landscape.
02 September 2025