Since their discovery in 2006, induced Pluripotent Stem Cells (iPSCs) have taken the world of stem cell biology by storm, providing new means to study the mechanisms of pluripotency, cancer biology, and aging. For the first time in history, iPSCs represent a cellular tool that can be used to unravel the mechanisms of human development and even to model disease. Moreover, these cells represent enormous potential in the development of cell-based therapies and could lead to the discovery of new or personalized pharmacological therapeutics to treat a wide variety of disorders.
Despite the inherent value of iPSCs, their first means of production were flawed, which made them challenging for clinical use. However, in 2010, REPROCELL became the first company to commercialize mRNA-based reprogramming technologies, the most reliable method for producing clinical-grade iPSCs. Today, REPROCELL employs its state-of-the-art StemRNA™ 4th Generation Reprogramming Technology to bring the power of mRNA reprogramming to the clinical sector.
What does mRNA reprogramming have to offer?
As a non-viral, non-retained method for the consistent, fast, and safe generation of human iPSC lines, mRNA reprogramming technology generates clinical-grade iPSCs from somatic tissue while avoiding the drawbacks of reprogramming methods using viruses or DNA vectors. Resultantly, this method has become widely acknowledged as the most highly productive, and best-suited footprint-free reprogramming system for the clinical production of iPSCs.
mRNA reprogramming offers:
- Low probability of genomic abnormalities generated during reprogramming.
- Rapid reprogramming kinetics, allowing for fully reprogrammed iPSCs in as few as 12 days.
- No retention of reprogramming vectors so that an extended culture to remove virus-based vectors is not required.
- High efficiency (greater than 1%) which enables the successful reprogramming of complex cells from diseased or elderly donors. In contrast, other methods yield reprogramming efficiencies varying from 0.00001 to 0.01%.
Understanding REPROCELL’s clinical process: The creation of iPSC Clones
REPROCELL’s Clinical iPSC project paradigm generates StemRNA Clinical iPSC Seed Clones as part of the process. The Sponsor can individually evaluate these clones to identify the optimal line to take forward into the Master Cell Bank (MCB) process. This assures that the Sponsor invests in the best and most suitable clone to generate their MCB, minimizing re-optimization risk at this later stage of generating a clinical product.
Furthermore, REPROCELL provides 4th Generation Seed iPSC Clones suited to their client’s needs, whether they require complete control of the donor characteristics or access to their existing bank of StemRNA Clinical iPSC Seed Clones. The bank offers rapid access to Seed Clones for proof of concept studies by the client and subsequent MCB development but also contains corresponding clone versions intended for research purposes only, called StemRNA Clinical iPSC Pilot Clones (Figure 1). When requested, additional vials are reserved for later GMP MCB manufacturing. Therefore, Pilot Clones are ideal for differentiation method development and to test their suitability for the Sponsor's project.
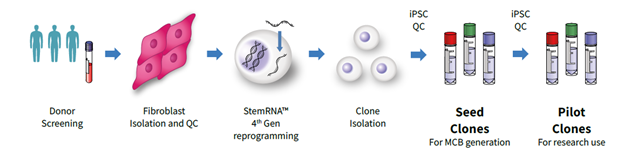
Figure 1: The StemRNA Clinical Process. Fibroblasts isolated by REPROCELL, or provided by the client, are reprogrammed to iPSCs using REPROCELLʼs own StemRNA 4th Gen reprogramming technology under CGTP guidelines. Multiple iPSC clones are isolated and quality controlled to create StemRNA Clinical iPSC Seed Clones, which are suitable for expansion into a GMP iPSC Master Cell Bank. The available research-grade StemRNA Clinical iPSC Pilot Clones are expanded from matching REPROCELLʼs off-the-shelf Seed Clones, subjected to quality control, and made available for research use only.
High standards for scientific quality
Clinical iPSC Seed Clones produced by REPROCELL are generated using highly controlled CGTP processes and are subject to rigorous quality control measures (Figure 2). As a GMP iPSC-Master Cell Bank manufacturer, REPROCELL adheres to the manufacturing and regulatory standards specified by key national regulatory agencies such as the US FDA, EMA, and PMDA Japan, allowing clients to access their target geography easily. Moreover, REPROCELL provides quality and regulatory documents, including COAs, batch records, and quality technical agreements.
With global access to human tissue samples, complete informed consent for clinical and commercial use, and full traceability of reagents, REPROCELL can provide you with the consistency, safety, and quality that your therapeutic project requires.
With years of experience generating iPSCs free of viruses and no remaining interferent vectors using mRNA reprogramming, REPROCELL can provide materials of the highest quality for your next scientific endeavor. Learn more about what REPROCELL can offer your project here.
| Test | Method | Specification |
| Mycoplasma testing | STAT Mycoplasma spp. (PCR) over 100 species | Negative |
| Sterility testing |
|
Negative |
| STR Genotyping | CellCheck 16 Plus (16 marker STR profile) |
STR profile of donor blood/fibroblasts/iPSC are identical |
| Quality and differentiation scoring my phase contract microscopy observation |
Quality Score A Diffirentiation score 0 |
|
| Viability | Trypan blue staining after thawing | 70% (1 million cells/vial) |
| Cell growth rate | Trypan blue staining at 1st/2nd passage | For information use only |
| Karyotype analysis | G-band analysis for 20 specimens | No karyotype abnormalities |
| Qualitative Pluripotency Marker Analysis | Immunofluorescence staining |
NANOG, Oct3/4, SSEA4; Postive SSEA1; Negative |
| Quantitive purity analysis/Identity validation | Flow cytometry analysis | NANOG ≥ 80% Oct3/4 ≥ 80% SSEA4 ≥ 70% |
| Differentiation capability | Directed differentiation and immunofluorescence | Capability to differentiate into all three germ layers |
Figure 2: QC Specifications for StemRNA™ Clinical iPSC Seed Clones and StemRNA Clinical iPSC Pilot Clones. REPROCELL offers simple and straightforward license terms for commercial and therapeutic use without any limitation of therapeutic areas of applications (as of Nov 2022).





.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



