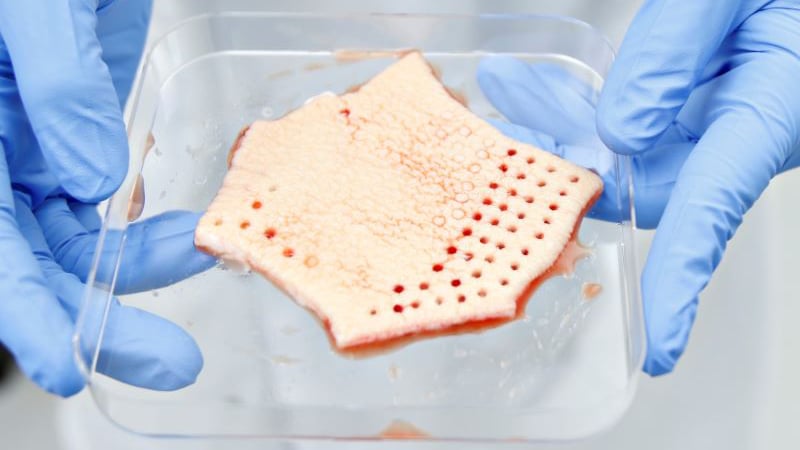
Preclinical and Drug Discovery CRO
REPROCELL Biopta’s human tissue technology predicts clinical success by using the closest possible model of drug behavior in humans.
Human tissue testing is a translational approach to drug discovery that bridges the gap between animal studies and clinical trials — increasing clinical success rates and adding commercial value to preclinical drug assets through proof of concept in patient-derived living tissues.
Types of human tissue assay
This invaluable information allows you to make ‘go’ or ‘no-go’ decisions with greater confidence, based on human data generated in fresh biospecimens sourced from the target patient population or in bioengineered human tissues. Find out more about our human tissue assays.
Alvetex® bioengineered 3D tissue services
Complementing our fresh tissue assays by accurately recreating human biology in bioengineered tissues.
Examples of our work
Our expertise in all areas of human tissue testing — including ethics, sourcing, handling, and fee-for-service experiments in human fresh tissue — allows us to act as your human tissue research department. See all our case studies.
Why use human tissue in drug discovery?
Managing risks and saving money
Human fresh tissue can be used to manage risk and save money across the entire drug discovery pipeline.
Demonstrating efficacy and safety
Human fresh tissue can be used in all phases of drug discovery programs, from target validation to clinical trouble shooting.

Our human tissue platform technologies
At REPROCELL, we offer a unique range of experimental techniques for translating data from preclinical species to humans. Explore our platform technologies.
Join our human tissue supplier network
We are investing in our tissue network to meet the growing safety and efficacy requirements of drug development programs and medicine regulators around the globe.
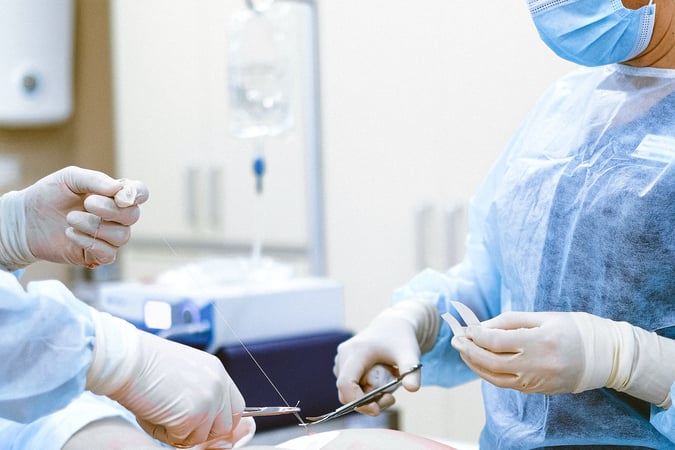
Clinical Laboratory Services and Precision Medicine
Laboratory services specialising in human biospecimens and AI-driven data analysis.
Clinical Laboratory Services
We coordinate, process, test, and analyze a range of fresh human biospecimens to meet the demands of your clinical research.

Pharmacology-AI
Machine learning platform for rapid identification of the features driving variation in patient outcomes — streamlining the development of effective patient stratification strategies.
On the REPROCELL blog
Latest in Drug Discovery

Modeling Fibrosis Progression Ex Vivo: Opportunities for Anti-Fibrotic Drug Screening
Explore the benefits of ex vivo human tissue models for fibrosis research and anti-fibrotic drug screening, offering a more accurate alternative to conventional preclinical methods.
15 January 2026

Enhancing Experimental Precision with the Alvetex® Advanced Culture System
Discover how Alvetex® Advanced enhances experimental precision with its flexible and functional 3D culture system for creating biologically accurate human skin models.
04 December 2025

Using Ex Vivo Skin Explants to Study Local Immune Responses in Psoriasis and Atopic Dermatitis
Explore the use of ex vivo skin explants to study local immune responses in chronic inflammatory skin diseases like psoriasis and atopic dermatitis.
17 November 2025

.jpg?width=800&height=600&name=REPROCELL-04.06.18_0460%20(1).jpg)