3D Bioengineered Inflammatory Bowel Disease Model
In vitro assays in 3D bioengineered tissues are increasingly used in the early stages of drug development to characterize lead compounds. The relevance of these models can vary greatly depending on how well they mimic the structure and function of human tissue. At REPROCELL, our in vitro 3D inflammatory bowel disease (IBD) tissue model is the most advanced commercially available bioengineered model of IBD. The tissues, which comprise immune cells, fibroblasts, and epithelial cells are bioengineered to mimic key features of the inflamed GI mucosa of IBD patients, allowing investigations into inflammation, barrier integrity, and fibrosis. These 3D tissues can be cultured for up to 96 hours, allowing prolonged or repeated exposure to test compounds. Test compounds can be applied using a prophylactic or therapeutic approach, and both acute or chronic treatments can be modelled. You can find out more below, view the publication, or contact our experts directly to book a free consultation.
What makes this in vitro system unique?
- Includes a viable immune component, mimicking infiltration of immune cells into the intestinal mucosa seen in IBD
- Stimulated to induce the inflammatory hallmarks of IBD using lipopolysaccharide (LPS) or cytokines
- Demonstrates the structural and functional effects of inflammation on the lamina propria compartment and overlying epithelium e.g., reduced barrier integrity, extracellular-matrix (ECM) remodeling
- Reflects the complex multicellular interactions of inflammation observed in IBD tissues

Cross-section showing the microscopic structure of the 3D IBD model before inducing an inflammatory response
What end-points can be measured in this model?
This model can be used to investigate the effects of your test articles on inflammation, barrier disruption, and ECM remodeling. Below we have listed some examples of endpoints that can be used to assess the therapeutic efficacy of your test compounds in IBD. If your pathway or end-point of interest is not listed below, our researchers can work with you to design a customized solution for your unique research needs.
Immune response
- Cytokine and chemokine production
- Inflammatory marker production
- Pro-inflammatory status
Barrier integrity
- Trans-epithelial resistance (TEER)
- Transport assays
- Histological assessment
- ICC/IHC staining
- Junction analysis
- Junction quantification by PCR and WB
- EM analysis
ECM remodelling
- ECM quantification
- ICC/IHC staining
- MMP/TIMP production
- Cytokine/chemokine production
- Marker quantification
What aspects of IBD can be modeled in this system?
This model mimics several of the molecular, cellular, and immunological features of IBD. For example, there is reduced trans-epithelial resistance (TEER) in the stimulated model, and evidence of hyperproliferation and pseudo-stratification of the epithelial layer, which matches observations made in IBD tissues.1,2 We also see upregulation of the following inflammatory mediators, pathways, and markers in this coculture system:
- Cytokines associated with Crohn's disease (TNFα, TNFβ, INFγ, IL-12p40, IL17A)3
- Chemokines associated with IBD (G-CSF, GM-CSF, GROa, CXCL9, CXCL10, MCP-1, MCP-3, RANTES)4
- COX2 cell signaling pathway5,6
- MMP-9 expression (a key biomarker for IBD)7
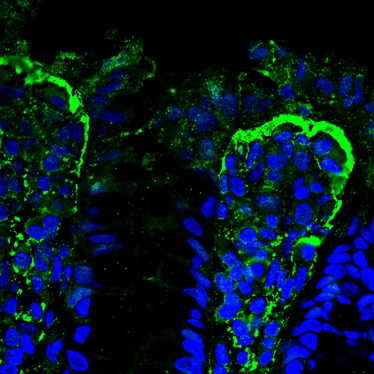
The IBD biomarker MMP-9 (green) accumulates at the epithelial/stromal interface of the 3D model, which is the same as can be observed in ulcerative colitis tissues of patients.
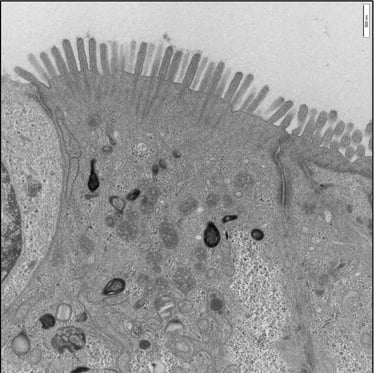
A confluent microvilli brush border is observed across the surface of the unstimulated 3D model, showing clear rootlet visibility.
What is included in an IBD project with REPROCELL?
- A customized project to meet your research goals.
- An expert Study Director to manage each project as your single point of contact from initiation to report.
- Ownership of all data generated during the project.
Measure the anti-inflammatory effects of your drug in a biologically relevant 3D model of IBD.
Assess the permeability of your IBD treatments in a biologically relevant 3D model of the human intestine.
References
- Gibson P et al. Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut 36:6 pp 857-863 (1995).
- Sarvestani SK et al. Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity. Nature Communications 12:262 (2021).
- Sanchez-Munoz F et al. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 21:14 pp 4280-4288 (2008).
- Miguel C et al. Chemokines and chemokine receptors in inflammatory bowel disease: Recent findings and future perspectives. Drug Discovery Today, 27:4 pp 1167-1175 (2022).
- Li Y et al. COX-2-PGE2 Signaling Impairs Intestinal Epithelial Regeneration and Associates with TNF Inhibitor Responsiveness in Ulcerative Colitis. EBioMedicine 36 pp 497-507 (2018).
- Spisni E et al. Cyclooxygenase-2 Silencing for the Treatment of Colitis: A Combined In Vivo Strategy Based on RNA Interference and Engineered Escherichia Coli. Molecular Therapy 23:2 pp 278-289 (2015).
- Baugh MD et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 117:4 pp 814-22 (1999).