Respiratory Assays
Our respiratory assays allow the safety or efficacy of your test drug to be determined in advance of clinical trials using human fresh intact lung tissues, including both healthy or diseased lungs.
For safety assessments, effects on respiratory function are required to be assessed as part of the ICH S7A core battery of tests prior to human exposure. Isolated airways provide an ideal model for the assessment of potential drug-mediated effects on airway resistance. Human ex vivo lung tissues also provide the optimum model for researching the effectiveness of your test articles in diseased lung tissues - for example, to assess bronchodilation or anti-inflammatory effects of new therapies.
Explore our respiratory assays
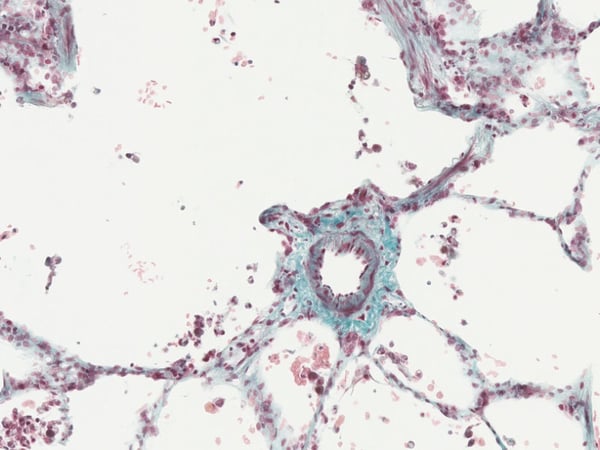
Precision-cut Lung Slices (PCLS)
A highly sought-after model for testing novel drugs, chemicals, xenobiotics, and cosmetics. By maintaining the structural integrity and functional properties of both human and animal lung tissues, PCLS provides a phenotypically accurate and relevant model for drug discovery and safety assessment. Our unique access to healthy and diseased lung tissue, including COPD and asthma donors, enables the investigation of drug effects across a range of respiratory conditions.

Pulmonary Artery Tone
The pulmonary arteries carry deoxygenated blood to the lungs and are essential for healthy respiratory function. Our organ bath model can measure changes in the vascular tone of these arteries in response to your test articles. This diverse test system can estimate a range of function endpoints ex vivo.

Airway Contractility
The airways of the respiratory tract offer resistance to airflow during inhalation and expiration. Any alterations to their structure – caused by contraction, increased mucus, or inflammation – can affect negatively airflow and gas exchange.
Secondary airways (healthy):
Secondary airways (diseased):
- Acetylcholine/carbachol model
- Adrenoceptor/salbutamol model
- Bronchodilation in secondary COPD human airways
Tertiary or lower airways:
Tertiary or lower airways (bronchodilation):
Tertiary or lower airways (diseased):
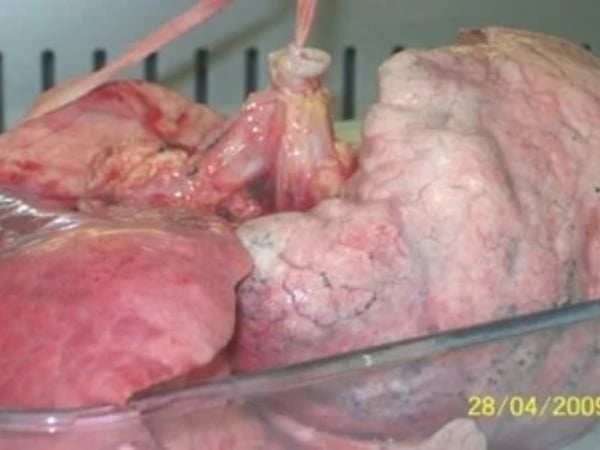
Lung Parenchyma Culture
Our parenchymal explant culture model provides an excellent platform to investigate and understand the mechanisms of lung diseases and the local effects of test compounds. These fresh explants contain the full complement of lung parenchymal cells in their native ratios and spatial orientation. This allows normal cell-cell communications and interactions to take place, replicating the local in vivo conditions of the lung.
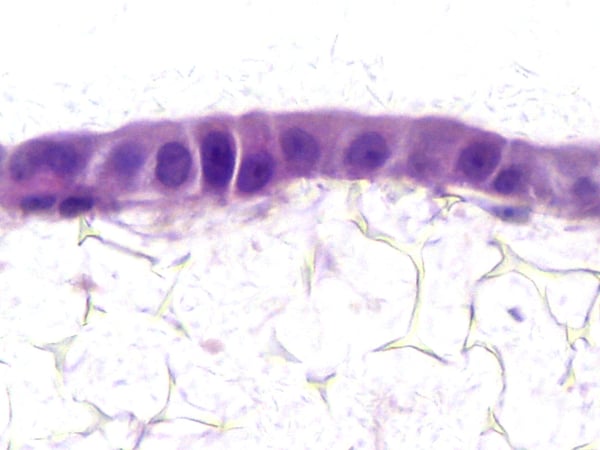
Bioengineered 3D Tissue Models: Human Lung
We provide the market's most clinically relevant assay for investigations of new drugs to treat Idiopathic Pulmonary Fibrosis (IPF), utilizing our 3D micro-physiological tissues built using Alvetex Scaffold, that closely reflect human IPF lung. Our service tests the efficacy of your drug within these disease-relevant models of IPF, presenting a comprehensive package tailored to meet your research requirements.
.jpg?width=756&height=425&name=Untitled%20design%20(10).jpg)

