Idiopathic Pulmonary Fibrosis (IPF) 3D Human Tissue Model
REPROCELL provides the market's most clinically relevant assay for investigations of new drugs to treat IPF, utilizing our 3D micro-physiological tissues that closely reflect human IPF lung. Our service tests the efficacy of your drug within these disease-relevant models of IPF, presenting a comprehensive package tailored to meet your research requirements. The tissues are built using REPROCELL's Alvetex scaffold, the market-leading technology for the creation of biologically-accurate human tissues, with over 130 peer-reviewed publications to date.
REPROCELL'S 3D in vitro IPF Tissue Model
Our IPF model is comprised of human primary lung fibroblasts isolated from patients with IPF, combined with bronchial epithelial cells, to create microphysiological lung tissues.
All scientists recognize the importance of structure in determining function. Creating a tissue that is truly reflective of human anatomy simply can't be rushed. Our Alvetex® technology enables cells to build a biologically-accurate tissue structure over multiple weeks, from which accurate function follows.
Why use REPROCELL’s IPF tissues to test potential new drugs?
REPROCELL’s CSO, Professor Stefan Przyborski, invented the Alvetex platform to enable the manufacture of complex human tissues that could greatly improve preclinical testing of drugs. Our IPF model consists of IPF-derived diseased lung fibroblasts and alveolar epithelial cells, to model the lung in health and disease. These individual tissues enable the assessment of multiple drugs and concentrations under various test conditions and exposure durations. As a result, they provide compelling evidence of efficacy in a human test system.
The IPF 3D tissue model is created in two stages. Firstly, the Alvetex scaffold is seeded with primary human lung fibroblasts and cultured for 2-4 weeks, during which endogenous extracellular matrix is laid down. Secondly, epithelial cells (A549 cells) are seeded on top of the fibroblast compartment and are cultured long term to encourage epithelial cell differentiation and maturation. A549 cells are used to recapitulate type II pneumocytes of the alveolar epithelium.


REPROCELL will test your compounds to provide insights into their efficacy or mechanism of action in a disease-relevant model of IPF.
A key aspect of REPROCELL's IPF model is the cross-talk between epithelial cells and fibroblasts, both during the creation of the tissue and any subsequent tests of drug efficacy conducted using the tissues. When compared to our healthy lung models (also available from REPROCELL), IPF disease models demonstrate enhanced ECM deposition, dysfunctional ECM remodelling, and elevated secretion of pro-fibrotic mediators, such as TGF-β1, PDGF and IL-8. IPF models also show an increase in fibroblast activation, as determined via αSMA (alpha smooth muscle actin) and FAP (fibroblast activation) expression.
REPROCELL’s 3D IPF model has been tested with the standard of care compounds pirfenidone and nintedanib, which can be used as comparators to your test compounds. Treatment with pirfenidone and nintedanib reduces total collagen and fibronectin content in diseased models. These drugs are currently used clinically to slow the progression of IPF.
Contact our scientists today to discuss how to compare your compounds to clinically-proven standard of care compounds, providing powerful proof of concept data in a human test system.
What is IPF and why are new drugs needed?
Idiopathic pulmonary fibrosis (IPF) is a degenerative, debilitating condition in which the lungs accumulate fibrotic scar tissue and respiratory function is lost. Although it is considered a rare disease, it is thought to affect over 3 million people worldwide; its incidence increases with age, typically affecting people over 70 years old.

IPF is localised to the alveolar epithelium and interstitium of the distal respiratory tract and can therefore be classified as an interstitial lung disease (ILD). Formerly thought to be driven by alveolar inflammation (alveolitis), IPF is now considered to be a fibroproliferative disorder, in which interstitial fibroblasts become activated and transdifferentiate into myofibroblasts. Such changes are associated with the pathological deposition of extracellular matrix proteins (ECM) and remodelling of the lung architecture.
Consequently, there is a thickening and stiffening of the interstitial space, leading to a reduction in gas exchange efficiency across the blood-air barrier, and gradual decline of respiratory function. Although several treatments can help reduce the rate at which IPF progresses, currently there are no therapeutic interventions that can successfully halt or reverse the disease.
Pathophysiology of IPF

The cause of the condition is unknown, making it challenging to target with pharmaceutical drugs. It is hypothesized that recurrent microinjuries to the alveolar epithelium, perhaps through prolonged exposure to environmental pollutants or to viruses, leads to the release of pro-fibrotic cytokines, including TGF-β1, TNF-⍺, IL-6, and IL-8. This, in turn, activates underlying fibroblasts, causing them to adopt a myofibroblast phenotype characterized by excessive extracellular matrix (ECM) deposition. There is an urgent need for new drug targets and treatments, which includes new tools to better predict drug efficacy during preclinical drug discovery. REPROCELL is one of a handful of research groups and companies working to develop new models and preclinical test systems, which can accelerate the development of new treatments for IPF.
Key Application: Testing Drug Reponses in Profibrotic Stimulation Protocols
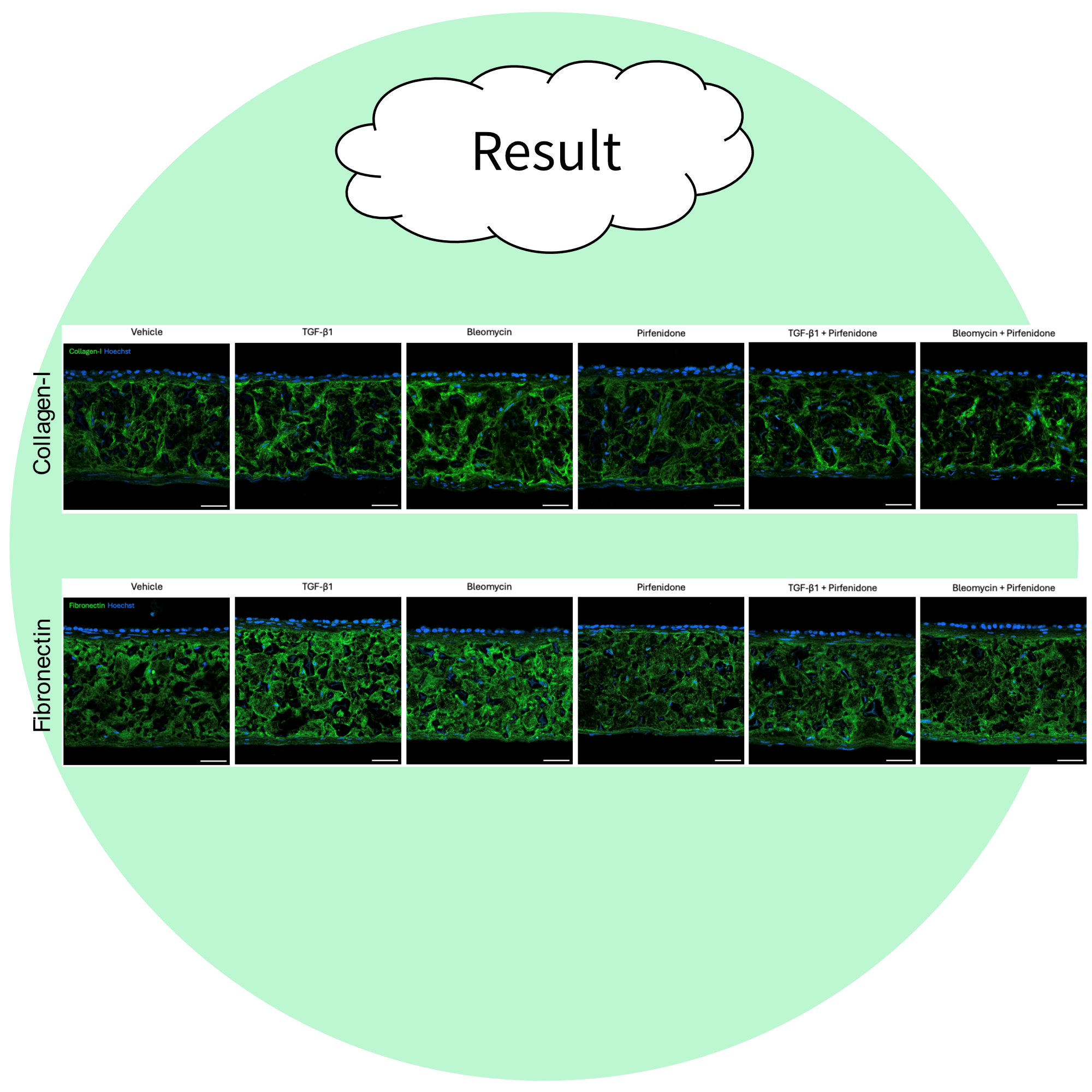
Total collagen and fibronectin is increased in the IPF lung models compared to the healthy controls (see below). In addition the effects of profibrotic stimuli such as TGF- β1 or bleomycin can be assessed in the model; both TGF-β1 and bleomycin are pro-fibrotic stimulants that are commonly used in models of IPF to initiate a fibrotic phenotype.

In our Alvetex model of IPF, TGF- β1 and bleomycin both lead to an increase in total collagen content in both healthy and diseased models, and alterations in the expression of matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP). Changes in the expression of MMP 1, MMP 3, and TIMP 2 in response to TGF-β1 or bleomycin in healthy and diseased lung models are shown below.

The effects of your test compound can be evaluated in the presence and/or absence of established profibrotic stimulators such as TGF-β1 or bleomycin (shown above) and can provide comparisons to the efficacy of standard of care drugs such as pirfenidone (below).

What is included in an IPF project with REPROCELL?
When you send your test compounds to REPROCELL Europe (UK), we'll assign a dedicated expert scientist, known as a Study Director, to collaborate closely with you, the Sponsor. Your Study Director will serve as your primary point of contact throughout the entire project, managing it from inception to completion. They will work with your team to design a tailored protocol that aligns with your research objectives, oversee the laboratory experiments, and deliver a comprehensive final report.
Contact us today to discuss how we can assist your innovative drug discovery programs.