Finding a suitable human iPSC (hiPSCs) line is the first step of many disease modeling and cell therapy projects. Once a suitable donor has been found and hiPSCs have been generated, certain quality control (QC) measures of the cell culture to verify its identity and quality are crucial. If portions of the QC are missing or deviate from the standard, the results acquired with these cells may be unusable or may produce misleading conclusions. Below we address common QC tests and why these are important. Here we focus on research-level projects, as clinical projects following regulatory guidelines require a much more strenuous, multilevel QC approach.
Sterility testing
Bacterial and fungal contamination are common complications in mammalian cell culture. This issue is even more complex as hiPSC culture media are not supposed to contain any antibiotics as it has been reported that continuous use of antibiotics can alter the gene expression profile of an iPSC line1. In consequence, using meticulously sterile techniques and additionally performing sterility assessment (by direct inoculation, or membrane filtration) is a good practice to ensure low-level bacterial contaminants are not present at the time of freezing the cells.
Mycoplasma testing
Mycoplasmas are small prokaryotic organisms that are resistant to antibiotics and can pass even through the smallest pore sizes of filters. They represent a huge problem in cell culture, as they cannot be detected under a microscope, and they do not overgrow a culture in a typical way like other common contaminants. Mycoplasma can remain in the culture undetected for a long time and spread to other cultures. These special bacteria can alter gene expression as well as induce karyotype abnormalities2,3, so it is crucial to verify that the culture is free of mycoplasma.
Cultures can become contaminated with mycoplasma via several routes, including contaminated culture reagents or contact with human skin. Different mycoplasma strains can be detected using 3 different methods: PCR, indirect staining, and agar and broth culture. Over 180 different species of mycoplasma have been inventoried and around 20 of them have been found to be common contaminants in cell culture indicating the importance of testing for the right strain of mycoplasma.
Human viral pathogen testing
Viral contamination of the culture can interfere with the normal differentiation and metabolism of the cell culture, as well as represent a hazard for laboratory workers and potential patients. Despite working with sterile techniques, for safety reasons, it is important to ensure that the cells have not been infected with human viral pathogens such as HIV or HCV. The exposure risk is low but can be easily be avoided by testing the cell lines. Human viral pathogen testing can be conducted on a blood sample of the donor at the time of tissue procurement via PCR and followed up with testing multiple times at different stages in the project using the iPSCs.
Pluripotency marker assessment
To confirm the pluripotency of the cells, it is good practice to check the expression levels of hiPSC hallmark genes, such as Nanog, Oct3/4, SSEA-4, TRA 1-60, and TRA 1-81. Several methods can be used to confirm their expression, and they differ in their sensitivity and whether the markers are being evaluated for quantity or just quality. Immunofluorescence staining and flow cytometry are the most common ones, but RT-PCR or Western Blotting are sometimes used as well.
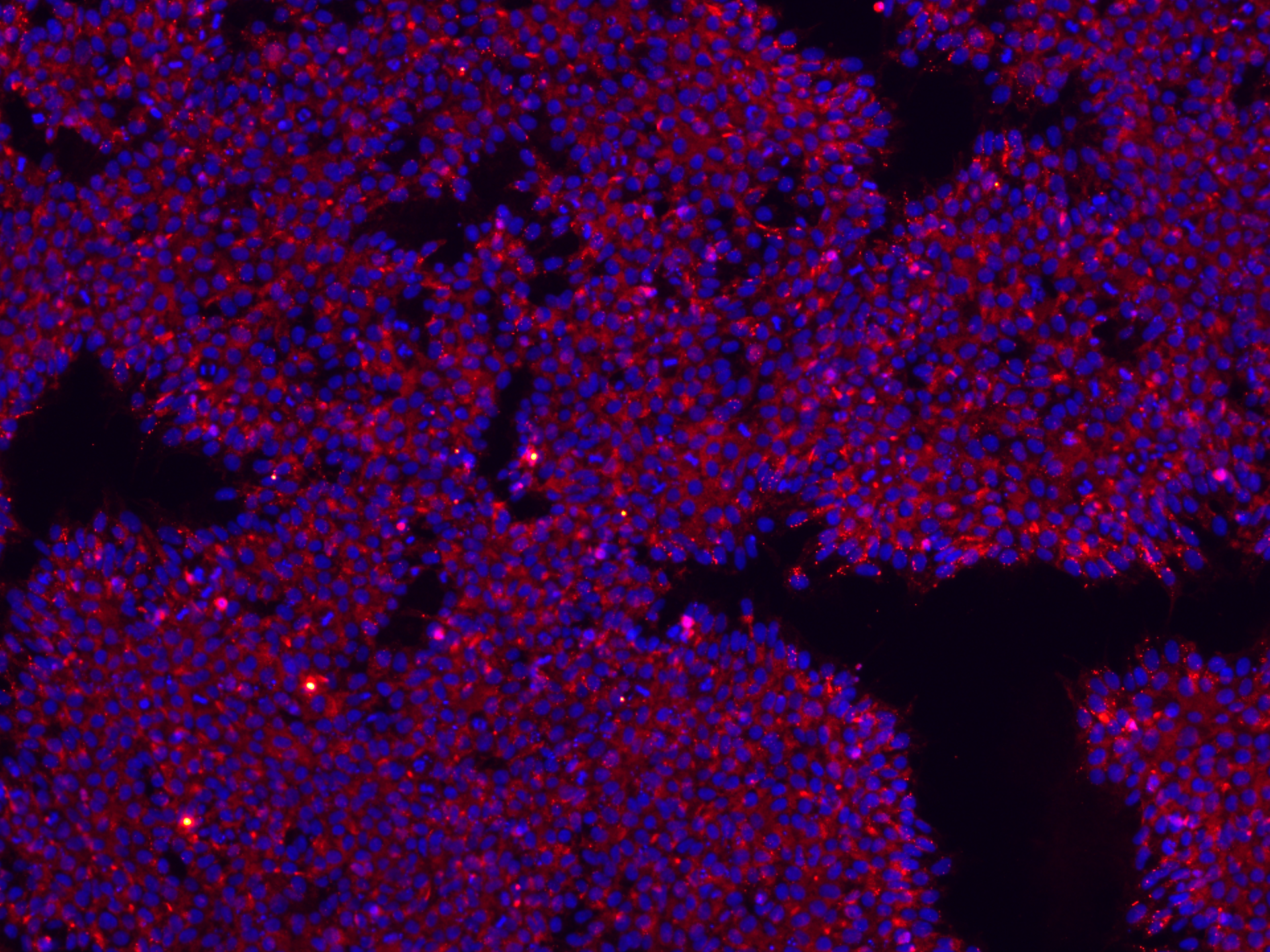
Figure 1. hiPSCs stained for pluripotency markers. Stains used recognize the TRA-1-60 cell surface antigen (red) and the hNanog nuclear transcription factor (blue).
Trilineage differentiation potential assessment
As a functional check of pluripotency, I strongly recommended to check the trilineage differentiation potential of any newly reprogrammed line. Every hiPSC should exhibit the potential to differentiate into ectoderm, mesoderm, and endoderm, or the cells cannot be qualified as pluripotent. Several methods can be used to determine trilineage capabilities; some are based on teratoma formation after injection of the cells into rodents, while others are based on directed differentiation of the hiPSCs or serum-based spontaneous differentiation. These pluripotency tests assess immature cells with either early germ marker expression or mature markers of specific cell types. The differentiation can be evaluated via immunohistochemistry, immunofluorescence, RT-PCR, or western blotting.
Karyotyping
Every cell line that is actively dividing and proliferating has the potential to accrue spontaneous mutation and genomic rearrangements, and stem cells are especially prone to this. Over time, different mutations (structural or functional) can arise. Most abnormalities will trigger the apoptosis mechanism, but some clones may develop a competitive growth advantage, and those can take over a culture within a couple of passages. Unfortunately, it also changes the metabolic and expression profile of the cell line. G-banding remains the gold standard for karyotyping, but supplementing it with digital PCR data and array CGH gives a better image of the genomic stability of a cell line.
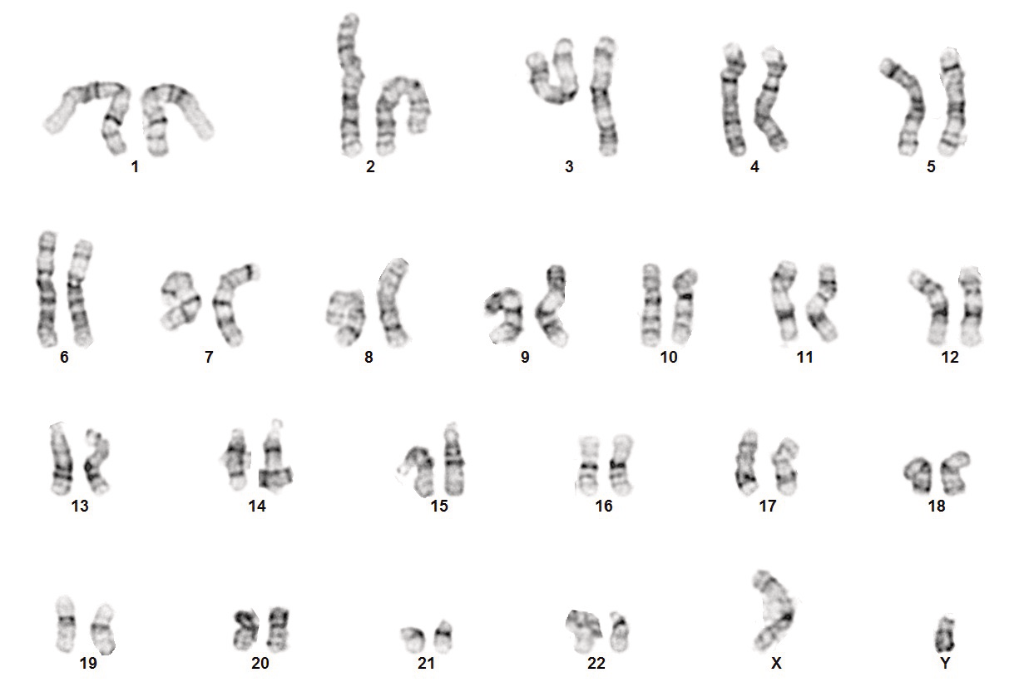
Figure 2. Normal G-band karyotyping results for hiPSCs from a normal male donor.
Sample authentication
When working with several lines in parallel, it is good practice to periodically keep track of and confirm the identity of each cell line. The most common assay for this is short tandem repeat (STR) analysis. This type of assay is based on the loci variability of specific genes in the DNA, which creates a unique profile for each cell line. To authenticate a hiPSC line sample one must make sure that the STR profile of the source tissue matches the downstream hiPSC clones. Of course, each clone as generated from the same donor starting material should have the same STR profile and cannot be distinguished with this type of assay.
A comprehensive quality control program with hiPSCs is crucial to ensure accurate, reproducible results, and it is important to see QC as a dynamic process that needs to reoccur periodically as opposed to just one time. Whatever the goals of your project, a robust QC testing protocol assessing sterility, pluripotency, and genetic abnormalities, while maintaining sample identity, is critical for gathering accurate data and ensuring high-quality results from experimental models.
References
1. Ryu AH, et al. Use antibiotics in cell culture with caution: Genome-wide identification of antibiotic-induced changes in gene expression and regulation. Scientific Reports 7:7533 (2017)
2. Fogh J, Fogh H. Karyotypic changes in mycoplasma-modified lines of FL human amnion cells. Proceedings of the Society for Experimental Biology and Medicine. 129:944 (1968).
3. Zhang S, Wear DJ, Lo S-C. Mycoplasmal infections alter gene expression in cultured human prostatic and cervical epithelial cells. Pathogens and Disease 27:43 (2000).



.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



