From Research to Clinical Translation
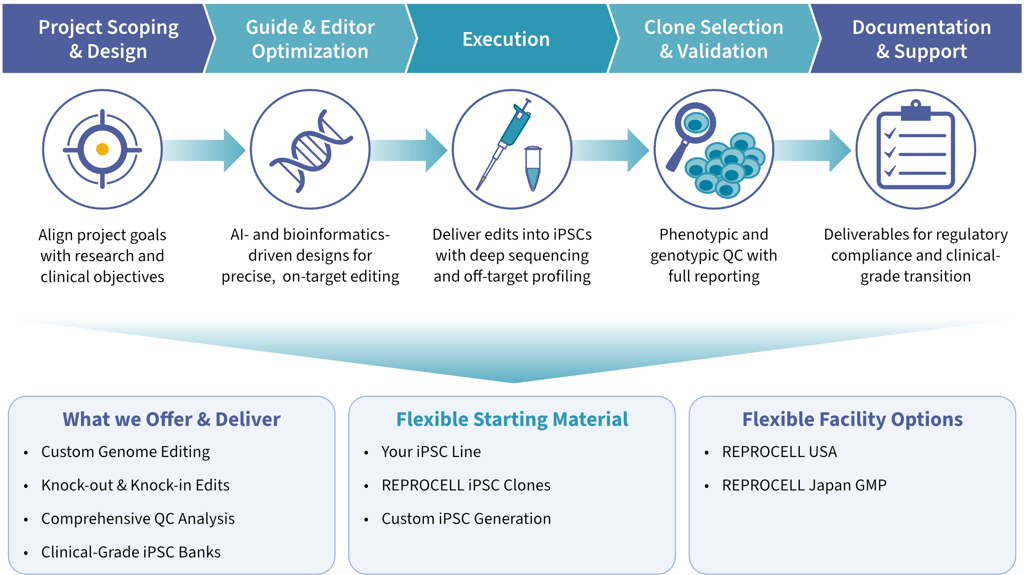
StemEdit is REPROCELL’s advanced genome editing platform for iPSCs, designed to support programs from early research through clinical development. REPROCELL uses this platform to enable partners to kick-start their gene-editing-based stem cell projects toward the clinic. Begin with research-grade editing and seamlessly transition to clinical-grade iPSC lines without restarting your cell line development.
AI-Designed Precision Editing
StemEdit leverages OpenCRISPR-1™, a de novo AI-designed gene editor created using large language models trained on extensive CRISPR-Cas datasets. The system is engineered for performance in human cells, and it demonstrates:
- High on-target editing efficiency
- Reduced off-target activity
- Lower predicted immunogenicity
These features are especially valuable in both preclinical research and clinical-stage programs, where precision and safety are critical.
OpenCRISPR-1™ is licensed from Profluent Bio. See press release, REPROCELL Launches StemEdit: Clinical Gene Editing Services and New iPSC Lines Using AI-Designed Editing System