- Clinical Stem Cell Services
- GMP iPSC/iMSC MCB Manufacturing – REPROCELL USA
Clinical Stem Cell Services
REPROCELL USA
GMP MCB Manufacturing Service
iPSCs and iMSCs for Therapeutic Use

At REPROCELL we specialize in the production of clinical iPSCs and iMSCs (iPSC-derived Mesenchymal Stem Cells) following FDA, EMA, and PMDA regulatory requirements while meeting the demands of therapeutic, pharmaceutical, and biotechnology companies at competitive prices.
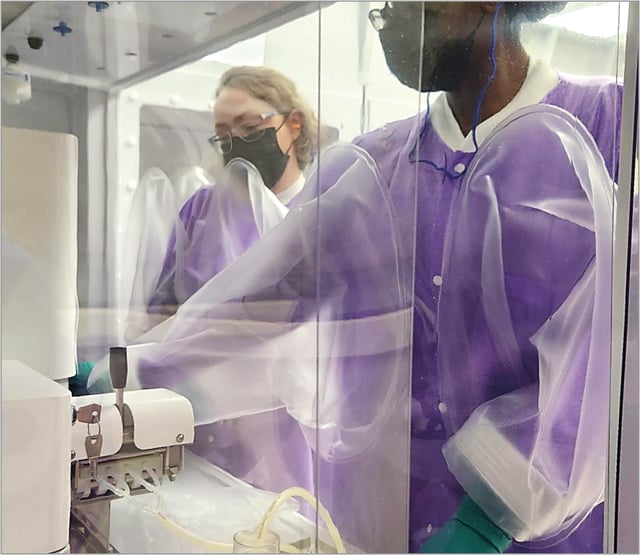
REPROCELLʼs state-of-the-art GMP facility in Beltsville, USA, features a closed cell culturing system enhancing stem cell manufacturing safety. This manufacturing site is equipped with multiple modules from the Cytocentric Xvivo System Model 2 from BioSpherix.

The Xvivo GMP system has a significantly lower contamination risk from room air and personnel compared to open clean room suites. It has a proven track record of producing a GMP-compliant environment for manufacturing iPSC and iMSC master cell banks (MCBs), suitable for therapeutic development and the production of other differentiated cell lineages. The system also integrates microscopes, centrifuges, and cryopreservation instruments. By minimizing contamination risks, it enhances cell product quality and accelerates turnaround times.
The Biospherix Cytocentric Xvivo system provides a GMP-grade cell production environment that meets Class 100 (ISO 5) standards through more than 120 HEPA-filtered air changes per hour. Its surrounding area is curtained and filtered to meet Class 10,000 (ISO 7). The system complies with the FDA Section 503B regulations, as well as ISO 14644 and USP 797 standards. Additionally, its environmental control and monitoring software follows 21 CFR Part 11, ensuring secure operator tracking and record management.

At REPROCELL we specialize in the production of clinically relevant iPSCs and iMSCs following FDA, EMA, and PMDA regulatory requirements while maintaining control over the entire clinical process, including:
Ethical donor recruitment & eligibility
Our donors are recruited in the US and the process follows FDA 21 CFR 1271, EMA COMMISSION DIRECTIVE 2006/17/EC and PMDA MHLW Notification No.375, along with an IRB approved informed consent form.
Rigorous selection of starting materials & reagents
Pathogen testing is performed at CLIA and EMA certified laboratories and we follow guidelines for USP29 <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products and EMA COMMISSION DIRECTIVE 2004/23/EC and PMDA MHLW Notification No.375 for raw materials.
Proprietary non-integrating StemRNA™ reprogramming technology
REPROCELLʼs StemRNA™ Clinical iPSCs are generated utilizing an in-house developed proprietary, footprint-free RNA reprogramming technology, eliminating the risk associated with retention of reprogramming vectors, and drastically enhancing the safety profile for clinical applications.

Ask our experts about our GMP iPSC and iMSC MCB production service
Discover more
Resources
- FAQ: Clinical iPSCs
- FAQ: Clinical MSCs
- FAQ: iPSC-Derived Exosomes
- Making iPSC-Derived Therapeutics a Clinical Reality – our external article in the European Biopharmaceutical Review.
Gene Editing Services