StemRNA™ 3rd Gen Reprogramming Kit
00-0076
Brand: StemRNA™
The StemRNA 3rd Gen Reprogramming Kit provides the fastest, most efficient method for generating clinically relevant iPS cells using a non-integrating, mRNA-based protocol. This technology supports generating iPSC lines derived from fibroblasts, blood, and urine using one multi-purpose kit.
Currency:
| Product name | Catalog number | Pack size | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|
| StemRNA™ 3rd Gen Reprogramming Kit | 00-0076 | 1 kit | (select above) | $ 1750.00 | £ 1434.00 | € 1676.00 |
Note: prices shown do not include shipping and handling charges.
Product Information
Recommended Products
- NutriStem™ hPSC XF Culture Medium for Human iPS and ES Cells 01-0005 / 01-0005-100
- iMatrix-511 Stem Cell Culture Substrate NP892-011 / NP892-012
- NutriFreez™ D10 Cryopreservation Medium 01-0020-50
Third Generation RNA Kit for Cellular Reprogramming of Fibroblasts, Blood, and Urine
The latest evolution in Stemgent RNA reprogramming, the StemRNA 3rd Gen Reprogramming Kit (formerly called the StemRNA-NM Reprogramming Kit), combines non-modified RNA and microRNA technology to provide a kit for stem cell researchers with a new level of versatility, simplicity and time savings, enabling cellular reprogramming of human fibroblasts, and cells from blood and now urine for difficult to reprogram patient samples.
Key Benefits
- Flexible technology generates high-quality human iPS cell lines from multiple target cell types
Out-of-the-box reprogramming of cells from skin (fibroblasts), blood (endothelial progenitor cells; EPCs) and urine (urine-derived progenitor cells; UPCs) - High efficiency, non-integrating reprogramming
StemRNA-3rd Gen requires a few as four additional reagents. Double stranded microRNAs enhance reprogramming efficiency, providing iPS cells with high efficiency (up to 4% from fibroblasts, up to 3% from EPCs, up to 0.5% from UPCs). - Time-saving protocol delivers faster results facilitating higher throughput
Colonies ready to pick in 10-14 days from fibroblasts and 12-16 days from EPCs or UPCs. No screening needed
| StemRNA-3rd Gen Reprogramming Kit (00-0076) | |||
|---|---|---|---|
| Feature | Fibroblasts | Urine (UPCs) | Blood (EPCs) |
| No. wells per kit | 9 | 3 | 3 |
| No Transfections required | 4 | 6-8 | 6-8 |
| Days to primary iPS cell colonies | 10-14 | 12-14 | 12-14 |
| Reprogramming efficiency | 2-4% | 0.1-0.5% | 0.4-3% |
| Screening Required | No | No | No |
| Xeno-compatible protocol | Yes | No | No |
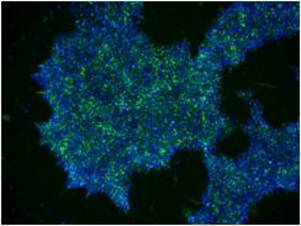
P7 primary iPS cells derived from UPCs were stained using the StainAlive TRA-1-60 Antibody (Cat. No. 09‑0068) and DAPI after expansion in NutriStem hPSC XF Medium (Cat. No. 01‑0005)
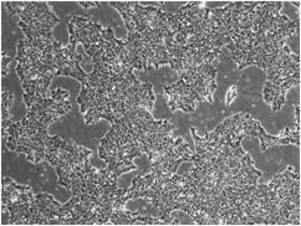
iPS cells derived from fibroblasts using the StemRNA-3rd Gen Reprogramming Kit were cultured on iMatrix-511 (Cat. No. NP892‑011) in NutriStem hPSC XF Medium (Cat. No. 01‑0005) for 7 passages. Magnification: 4x
 Timeline for StemRNA 3rd reprogramming of fibroblasts
Timeline for StemRNA 3rd reprogramming of fibroblasts
Download our StemRNA-3rd Gen Reprogramming Posters:

Stemgent and the StemRNA brand name are trademarks of REPROCELL Inc., Japan.
Product Name: StemRNA 3rd Gen Reprogramming Kit
Catalog Number: 00-0076
Storage and Stability: Store all three kit components at or below −70 °C. Kit components are stable for a minimum of 3 months from date of receipt when stored as directed.
Quality Control: The individual mRNAs are tested for size and integrity. The StemRNA 3rd Gen Reprogramming Kit is functionally validated for successful RNA-based reprogramming of adult fibroblasts, human umbilical vein endothelial cells (HUVECs), EPCs, and UPCs. Complete reprogramming of iPS cell colonies is confirmed by expression of pluripotency markers and appropriate colony morphology. All components of the kit are sterile and have tested negative for Mycoplasma spp.
Recommended Usage: For use with associated StemRNA 3rd Gen Reprogramming Kit protocols for generation of iPS cells.
Kit Contents:
- OKSMNL NM-RNA (Part No. 05-0040), 30 µg, 1 vial
- EKB NM-RNA (Part No. 05-0041), 20 µg, 1 vial
- NM-microRNAs (Part No. 05-0042), 15 µg, 1 vial
Brochure:
Specification Sheets:
Safety Data Sheets:
Protocols:
Posters:
FAQ:
Featured Publication
The StemRNA 3rd Gen Reprogramming Kit was used to generate iPSCs from fibroblasts in a microfluidic system that used only 1500 cells in 20 µL of medium. RNA-based reprogramming is ideal for such an application since it does not require extended culture for the elimination of reprogramming vectors.
Additional Publications
- Nagawa M; Nogi M; Doi H; Ohno H; Mochizuki M; Hayashi K'' Saito H. MDM4 enables efficient human iPS cell generation from PBMCs using synthetic RNAs. Research Square :https://doi.org/10.21203/rs.3.rs-6250001/v1 (2025).
- Jiang Y; Devito LG; Muntoni F; Healy L; Tedesco FS. Generation of a human induced pluripotent stem cell line (CRICKi021-A) from a patient with Ullrich congenital muscular dystrophy carrying a pathogenic mutation in the COL6A1 gene. Stem Cell Research 83:103648 (2025).
- Zetterdahl OG; Crowe JA; Reyhani S; Güra MA; Labastida-Botey O; Girard AS; Froese DS; Ahlenius H; Canals I. Generation of iPSC Lines with Tagged α-Synuclein for Visualization of Endogenous Protein in Human Cellular Models of Neurodegenerative Disorders. eNeuro 12:https://doi.org/10.1523/ENEURO.0093-25.2025 (2025).
- Doğan A; Şenkal-Turhan S; Bulut-Okumuş E;. Neuromesodermal progenitor derived mesenchymal stem cells: A new source for osteogenesis and adipogenesis in vitro. Biochimie 236:104 (2025).
- Kaur N; Singh J. Generation and characterization of human iPSC-derived astrocytes with potential for modeling X-linked adrenoleukodystrophy phenotypes. Int J Molecular Sci 26:1576 (2025).
- Huang T; Radley A; Yanagida A; Ren Z; Carlisle F; Tahajjodi S; Kim D; O'Neill P; Clarke J; Lancaseter MA; Heckhausen Z; Zhuo J; de Sousa JPA; Hajkova P; von Meyenn F; Imai H; Nakauchi H; Guo G; Smith A; Masaki H; . Inhibition of PRC2 enables self-renewal of blastoid-competent naive pluripotent stem cells from chimpanzee. Cell Stem Cell 32:P627 (2025).
- Isla-Magarané H; Zifiaurre-Seijo M; Zapata MA; Carcía-Arumí J; Duarri A. Generation of three human induced pluripotent stem cell lines from retinitis pigmentosa 25 patient and two carriers but asymptomatic daughters. Stem Cell Res 82:103645 (2025).
- Shomer I; Mor N; Raviv S; Budick-Harmelin N; Matchevich T; Avkin-Nachum S; Rais Y; Haffner-Krausz R; Haimovich A; Ziv A; Fluss R; Ben-Ze'ev B; Heimer G; Silachev DN; Katanaev VL; Dominissina D. Personalized allele-specific antisense oligonucleotides for GNAO1-neurodevelopmental disorder. Mol Therap Nucleic Acids 36:https://doi.org/10.1016/j.omtn.2024.102432. (2024).
- Schottmann NM; Klug K; Klopcki E; Üçeyler. Generation of induced pluripotent stem cell line (UKWNLi008) derived from a patient carrying a c.1678C>G variant in the transient receptor potential cation channel subfamily A member (TRPA1) gene potentially associated with small fiber neuropathy. Stem Cell Res 69:103094 (2023).
- Jeriha J; Kolundzic N; Khurana P; Perez-Dominguez A; Ilic D. mRNA-based reprogramming under xeno-free and feeder-free conditions. Meth Mol Biol 2454:665 (2020).
- Alowaysi M; Astro V; Fiacco E; Alzahrani F; Alkuraya FS; Adamo A. Generation of iPSC lines (KAUSTi011-A, KAUSTi011-B) from a Saudi patient with epileptic encephalopathy carrying homozygous mutation in the GLP1R gene. Stem Cell Res 50:1021 (2021).
- Patananan AN; Sercel AJ; Wu T-H; Ahsan FM; Torres Jr. A; Kennendy SAL; Vandiver A; Collier AJ; Mehrabi A; Van Lew J; Zakin L; Rodriguez M; Sixto M; Tadros W; Lazar A; Sieling PA; Nguyen T: Dawson ER; Braas D; Golovato J; Cisneros L; Vaske C; Plath K; Rabizadeh S; Niazi KR; Chiou P-Y; Teitell MA. Pressure-Driven Mitochondrial Transfer Pipeline Generates Mammalian Cells of Desired Genetic Combinations and Fates. Cell Reports 33:108562 (2020).
- Gurusamy N; Rajasingh S; Sigamani V; Rajasingh R; Isai DG; Czirok A; Bittel D; Rajasingh J. Noonan syndrome patient-specific induced cardiomyocyte model carrying SOS1 gene variant c.1654A>G. Exp Cell Research 400:112508 (2021).
- Yang D; Patel S; Szlachcic J; Scaduto S; Putluri N; Sreekumar A; Suliburk J; Metzker M; Balasubramanyam A; Borowiak M. Pancreatic Differentiation of Stem Cells Reveals Pathogenesis of a Syndrome of Ketosis-Prone Diabetes. Diabetes 70:2419 (2021).
- Yang J-Y; Lu B; Feng Q; Alfaro JS; Chen P-H; Loscalzo J; Wei W-B; Zhang Y-Y; Lu S-J; Wang S. Retinal Protection by Sustained Nanoparticle Delivery of Oncostatin M and Ciliary Neurotrophic Factor Into Rodent Models of Retinal Degeneration. Translational Vision Sci Tech 10:6 (2021).
- Sekiya A; Takasawa K; Arai Y; Horike S-I; Akutsu H; Umezawa A; Nishio K. Variation of DNA methylation on the IRX1/2 genes is responsible for the neural differentiation propensity in human induced pluripotent stem cells. Regen Therapy 21:620 (2022).
- Akiyama T; Sato S; Ko SBH; Sano O; Sato S; Saito M; Nagai H; Ko MSH; Iwata H. Synthetic mRNA-based differentiation method enables early detection of Parkinson's phenotypes in neurons derived from Gaucher disease-induced pluripotent stem cells. Stem Cell Transl Med 10:572 (2020).
- Beyer M; Klein T; Klug k; Klopocki E; Üçeyler N. Generation of the induced pluripotent stem cell line UKWNLi005-A derived from a patient with the GLA mutation c.376A>G of unknown pathogenicity in Fabry disease. Stem Cell Res 61:102747 (2022).
- Morita K; Nakamura A; Machida M; Kawasaki T; Nakanishi R; Ichida J; Iwata A; Akutsu H. Efficient reprogramming of human fibroblasts using RNA reprogramming with DAPT and iDOT1L under normoxia conditions. Regen Therapy 21:389 (2022).
- Drouin-Ouellet J; Legault EM; Nilsson F; Pircs K ;Bouquety J; Petit F; Shrigley S; Birtele M; Pereira M; Storm P; Sharma Y; Bruzelius A: Vuono R; Kele M; Stoker TB; Ottosson DR; Falk A; Jakobsson J; Barker RA; Parmar M. Age-related pathological impairments in directly reprogrammed dopaminergic neurons derived from patients with idiopathic Parkinson’s disease. Stem Cell Rep 17:2203 (2022).
- Devito LG; Cooper F; D'Angelo I; Smith J; Healy L. Generation of FOUR iPSC lines (CRICKi004-A; CRICKi005-A; CRICKi006-A, CRICKi007-A) from Spinal muscle atrophy patients with lower extremity dominant (SMALED) phenotype. Stem Cell Res 65:102954 (2022).
- Seah C; Breen MS; Rusielewicz; Bader HN; Xu C; Hunter CJ; BcCarthy B; Deans PJM; Chattopadhyay; M; Goldberg J; Desarnaud F; Makotkine I; Flory JD; Beirer LM; StaniskyteM; Noggle SA; Huckins LM; Paull D; Brennand KJ; Yehuda R. Modeling gene × environment interactions in PTSD using human neurons reveals diagnosis-specific glucocorticoid-induced gene expression. Nature Neurosce :https://doi.org/10.1038/s41593-022-01161-y (2022).
- Supakul S; Leventoux N; Tabuchi J; Mimura M; Ito D; Maeda S; Okano H. Establishment of KEIOi005-A iPSC line from urine-derived cells (UDCs) of a mild Alzheimer’s disease (AD) donor with multiple risk SNPs for sporadic Alzheimer’s disease (sAD). Stem Cell Res 62:102802 (2022).
- Iuso A; Zhang F; Rushi E; Campbell B; Dorn T; Zanuttigh E; Haas D; Anikster T; Lederer G; Pertek A; Nteli P; Laugwitz K-L; Moretti A. Generation of two human iPSC lines, HMGUi003-A and MRIi028-A, carrying pathogenic biallelic variants in the PPCS gene. Stem Cell Res 61:102773 (2022).
- Zorzan I; Gagliano O; Elvassore N; Martello G. Using Microfluidics to Generate Human Naïve and Primed Pluripotent Stem Cells. In: Rugg-Gunn P. (eds) Human Naïve Pluripotent Stem Cells.. Meth Mol Biol 2416:doi.org/10.1007/978-1-0716-1908-7_5 (2021).
- Bifani AM; Tan HC; Choy MM; Ooi EE. Cell Strain-Derived Induced Pluripotent Stem Cells as an Isogenic Approach To Investigate Age-Related Host Response to Flaviviral Infection. J Virol 96:e01737-21 (2022).
- Rajasingh S; Sigamani V; Selvam V; Gurusamy S; Kirankumar S; Vasanthan J; Rajasingh J. Comparative analysis of human induced pluripotent stem cell-derived mesenchymal stem cells and umbilical cord mesenchymal stem cells. J Cell Mol Med 25:8904 (2021).
- Fiacco E, Alowaysi M; Astro V; Adamo A. Generation of an iPSC cohort of isogenic iPSC lines (46-XY and 47-XXY) from a non-mosaic Klinefelter Syndrome patient (47-XXY) (KAUSTi008-A, KAUSTi008-B, KAUSTi008-C, KAUSTi008-D, KAUSTi008-E, KAUSTi008-F, KAUSTi008-G). Stem Cell Res 50:102119 (2021).
- Fiacco E; Alowaysi M; Astro V; Adamo A. Derivation of two naturally isogenic iPSC lines (KAUSTi006-A and KAUSTi006-B) from a mosaic Klinefelter Syndrome patient (47-XXY/46-XY). Stem Cell Res 49:102049 (2020).
- Alowaysi M; Fiaccdo E; Astro V; Adamo A. Establishment of an iPSC cohort from three unrelated 47-XXY Klinefelter Syndrome patients (KAUSTi007-A, KAUSTi007-B, KAUSTi009-A, KAUSTi009-B, KAUSTi010-A, KAUSTi010-B). Stem Cell Res 49:102042 (2020).
- Isono W; Kawasaki T; Ichida JK; Ayabe T; hiraike O; Umezawa A; Akutsu H. The combination of dibenzazepine and a DOT1L inhibitor enables a stable maintenance of human naïve-state pluripotency in non-hypoxic conditions. Regen Therap 15:161-168 (2020).
- Alowaysi M; Fiacco E; Astro V; Adamo A. Establishment of iPSC lines from a high-grade Klinefelter Syndrome patient (49-XXXXY) and two genetically matched healthy relatives (KAUSTi003-A, KAUSTi004-A, KAUSTi004-B, KAUSTi005-A, KAUSTi005-B, KAUSTi005-C). Stem Cell Res 49:102008 (2020).
- Zorzan I; Pellergrini M; Arboit M; Incarnato I; Maldotti M; Forcato M; Malagoli Tagliazucci G; Carbognin E; Montagner M; Oliciero S; Martello G. The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs. Nature Commun 11:2364 (2020).
- Grosch M; Ittermann S; Rusha E; Greisle T; Ori C; Truong D-JJ; O'Niell AC; Pertek A; Westmeyer GG; Drukker M. Nucleus size and DNA accessibility are linked to the regulation of paraspeckle formation in cellular differentiation. BMC Biology 18:42 (2020).
- Wamaitha SE; Grybel KJ; Alanis-Lobato G; Gerri C; Ogushi S; McCarthy A; Kahdevaiah SK; Healy L; Lea RA; Molina-Arcas M; Devito LG; Elder K; Snell P; Christie L; Downward J; Turner JMA; Naikan KK. IGF1-mediated human embryonic stem cell self-renewal recapitulates the embryonic niche. Nature Commun 11:764 (2020).
- Gong J; Cai H; NYSCF Global Stem Array Team; Noggle S; Paull D; Rizzolo LJ; Del Priore LV; Fields MA. Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions. Stem Cells Transl Med :https://doi.org/10.1002/sctm.19-0321 (2019).
- Liu G; David BT; Trawczynski M; Fessler RG. Advances in pluripoptent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev Rep :doi.org/10.1007/s12015-019-09935-x (2019).
- Bredenkamp N; Yang J; Clarke J; Stirparo GG; von Meyenn F; Baker D; Drummond R; Ren Y; Li D; Wu C; Rostovskaya M; Eminli-Meissner S; Smith A; Guo G. Wnt Inhibition Facilitates RNA-Mediated Reprogramming of Human Somatic Cells to Naive Pluripotency. Stem Cell Rep 13:1083 (2019).
- Nakajima M; Yoshimatsu S; Sato T; Nakamura M; Okahara J; Sasaki E; Shiozawa S; Okano H. Establishment of induced pluripotent stem cells from common marmoset fibroblasts by RNA-based reprogramming. Biochem Biophys Research Commun in press:https://doi.org/10.1016/j.bbrc.2019.05.175 (2019).
- Watanabe T; Yamazaki S; Yoneda N; Shinohara H; Tomioka I; Iiguchi T; Tagoto M; Ema M; Suemizu H; Kawai K; Sasaki E. Highly efficient induction of primate iPS cells by combining RNA transfection and chemical compounds. Genes to Cells in press:doi:10.1111/gtc.12702 (2019).
- Liu L-P; Li Y-M; Guo N-N; LI S; Ma X; Zhang Y-X; Gao Y; Huang J-L; Zheng D-X; Wang L-Y; Xu H; Hui L; Zheng Y-W. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Reports 27:455-466.e5 (2019).
- Sacco AM; Belviso I; Romano V; Carfora A; Schonauer F; Nurzynska D; Montagnani S; Di Meglio F; Castaldo C. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J Cell Mol Med :1-13; https://doi.org/10.1111/jcmm.14316 (2019).
- Klein T; Klug K; Henkel L; Kwok CK; Edenhofer F; Klopocki E; Kurth I; Üceyler N. Generation of two induced pluripotent stem cell lines from skin fibroblasts of sisters carrying a c.1094C>A variation in the SCN10A gene potentially associated with small fiber neuropathy. Stem Cell Res 35:101396 (2019).
- Dasgupta B; Rusha E; Drukker M. iPSC generation, prime to naïve reversion & characterization and primordial germ cell differentiation of Northern White Rhino. Univ Bremen : (2019).
- Su S; Guntur AR; Nguyen DC; Fakory SS; Doucette CC; Leech C; Lotana H; Kelley M; Kohil J; Martino J; Sims-Lucas S; Liaw L; Vary C; Rosen CJ; Brown AC. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell Reports 25:3215-3228.e9 (2018).
- Klein T; Henkel L; Klug K; Kwok CK; Klopocki E; Üceyler N. Generation of the human induced pluripotent stem cell line UKWNLi002-A from dermal fibroblasts of a woman with a heterozygous c.608 C>T (p.Thr203Met) mutation in exon 3 of the nerve growth factor gene potentially associated with hereditary sensory and autonomic neuropathy type 5. Stem Cell Research https://doi.org/10.1016/j.scr.2018.10.017 (2018)
- Liu X; Nefzger CM; Rossello FH; Chen J; Knaupp AS; Firas J; Ford E; Pflueger J; Paynter JM; Chy HS; O'Brien CM; Huang C; Mishra K; Hodgson-Garms M; Jansz N; Williams SM; Blewitt ME; Nilsson SK; Schittenhelm RL; Laslett AL; Lister R; Polo JM. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nature Methods 14:1055 (2017)