NutriStem™ hPSC XF Culture Medium for Human iPS and ES Cells
01-0005 / 01-0005-100
Brand: NutriStem™
NutriStem hPSC XF Culture Medium is a fully-defined, xeno-free, low growth factor concentration, feeder-free culture medium for human embryonic stem (ES) and induced pluripotent stem (iPS) cells. Cells can be cultured for at least 20 passages while retaining pluripotency marker expression, robust proliferation with a normal karyotype, and the ability to differentiate into cells of all three germ layers in vitro and in vivo.

Currency:
| Product name | Catalog number | Sartorius Code | Pack size | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|---|
| NutriStem™ hPSC XF Culture Medium (500 mL) | 01-0005 | 05-100-1A | 500 mL | (select above) | $ 517.00 | £ 305.00 | € 353.00 |
| NutriStem™ hPSC XF Culture Medium (100 mL) | 01-0005-100 | 05-100-1B | 100 mL | (select above) | $ 136.00 | £ Ask us | € Ask us |
Note: prices shown do not include shipping and handling charges.
Product Information
Notice to clinical users: A Drug Master File for NutriStem medium has been filed with the US FDA. Please contact us for more information.
NutriStem hPSC XF Medium (formerly called NutriStem XF/FF Culture Medium) is a fully defined xeno-free, low growth factor human embryonic stem (ES) and induced pluripotent stem (iPS) cell feeder-free culture medium that enables maintenance and expansion of pluripotent stem cells. NutriStem Medium can be used to culture pluripotent stem cells for at least 20 passages while retaining pluripotency marker expression, robust proliferation with a normal karyotype, and the ability to differentiate into cells of all three germ layers in vitro and in vivo. NutriStem Medium offers the ability to culture cells in a completely xeno-free medium without the need for high levels of basic FGF and other stimulatory growth factors and cytokines. In addition, the superior cell attachment and proliferation observed with NutriStem Medium aid high-throughput screening applications.
- Xeno-free, feeder-free conditions contain no animal components
- Easy, one-step transition from feeder-dependent culture, no adaptation required
- Maintains pluripotency, normal morphology, karyotype, and differentiation potential of human ES and iPS cells over long term culture
- Robust attachment and high cloning efficiency from single cells
-
Amenable to a weekend-free culture
Morphology of Human H1 ES cells Grown in NutriStem hPSC XF Medium for 20 passages.
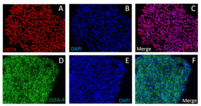
Expression of Pluripotency Markers in hES Cells Cultured in NutriStem hPSC XF medium.
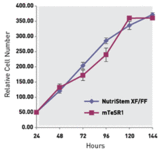 Proliferation Growth Rate of Human H1 ES cells Cultured in NutriStem hPSC XF Medium versus Competitor Medium.
Proliferation Growth Rate of Human H1 ES cells Cultured in NutriStem hPSC XF Medium versus Competitor Medium.
The Sartorius company name and logo are the property of Sartorius GmbH, Germany.
Product Name: NutriStem hPSC XF Medium
Catalog Number
- 01-0005 (Sartorius Cat # 05-100-1A): 500 mL
- 01-0005-100 (Sartorius Cat # 05-100-1B): 100 mL
Storage and Stability: Store at −20°C. This product is stable for a minimum of 3 months when stored as directed. See product label for lot-specific expiration date
Quality Control: NutriStem Medium tested negative for mycoplasma. Human pluripotent stem cells expressed the pluripotency markers SSEA-4, TRA-1-60, and TRA-1-81 after three continuous passages in NutriStem Medium
Sterility: Sterile
Notice To Purchaser: REPROCELL is an authorized global distributor of select cell culture products from Sartorius AG (Germany).
Manufacturer: Biological Industries, a subsidiary of Sartorius GmbH (Germany)
Related Products:
- StemRNA 3rd Gen Reprogramming Kit
- NutriFreez D10 Freezing Medium
Specification Sheets:
Safety Data Sheets:
Product Flyers:
Protocols:
Application Notes:
- Jiang Y; Devito LG; Muntoni F; Healy L; Tedesco FS. Generation of a human induced pluripotent stem cell line (CRICKi021-A) from a patient with Ullrich congenital muscular dystrophy carrying a pathogenic mutation in the COL6A1 gene. Stem Cell Research 83:103648 (2025).
- Devito LG; Lionello VM; Muntoni F; Tedesco FS; Healy L. Generation of an MTM1-mutant iPSC line (CRICKi008-A) from an individual with X-linked myotubular myopathy (XLMTM). Stem Cell Res 69:102079 (2023).
- Clemens DJ; Ye D; Wang L; Kim CSJ; Zhou W; Dotzler SM; Tester DJ; Marty I; Knollmann BC; Ackerman MH; Cellular and electrophysiological characterization of triadin knockout syndrome using induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Reports 18:1075 (2023).
- Shimada M; Tokumiya T; Miyake T; Tsukada K; Kanzaki N; Yanagihara H; Kobayashi J; Matsumoto Y. Implication of E3 ligase RAD18 in UV-induced mutagenesis in human induced pluripotent stem cells and neuronal progenitor cells. J Radiation Res 2023:1 (2023).
- Devito LG; Zanjani ZS; Evans JR; Scardamaglia A; Houlden H; Gandhi S; Healy L. Generation of TWO G51D SNCA missense mutation iPSC lines (CRICKi011-A, CRICKi012-A) from two individuals at risk of Parkinson's disease. Stem Cell Res 71:103134 (2023).
- Devito LG; Cooper F; D'Angelo I; Smith J; Healy L. Generation of FOUR iPSC lines (CRICKi004-A; CRICKi005-A; CRICKi006-A, CRICKi007-A) from Spinal muscle atrophy patients with lower extremity dominant (SMALED) phenotype. Stem Cell Res 65:102954 (2022).
- Miyake T; Kuge M; Matsumoto Y; Shimada M. α-glucosyl-rutin activates immediate early genes in human induced pluripotent stem cells. Stem Cell Res 56:102511 (2021).
- Alowaysi M; Astro V; Fiacco E; Alzahrani F; Alkuraya FS; Adamo A. Generation of iPSC lines (KAUSTi011-A, KAUSTi011-B) from a Saudi patient with epileptic encephalopathy carrying homozygous mutation in the GLP1R gene. Stem Cell Res 50:1021 (2021).
- Fiacco E; Alowaysi M; Astro V; Adamo A. Derivation of two naturally isogenic iPSC lines (KAUSTi006-A and KAUSTi006-B) from a mosaic Klinefelter Syndrome patient (47-XXY/46-XY). Stem Cell Res 49:102049 (2020).
- Alowaysi M; Fiaccdo E; Astro V; Adamo A. Establishment of an iPSC cohort from three unrelated 47-XXY Klinefelter Syndrome patients (KAUSTi007-A, KAUSTi007-B, KAUSTi009-A, KAUSTi009-B, KAUSTi010-A, KAUSTi010-B). Stem Cell Res 49:102042 (2020).
- Nowak-Imialek M; Wunderlich S; Herrmann D; Breitschuh-Leibling S; Cohring C; Petersen J; Klein S; Baulain U; Lucas-Hahn A; Martin U; Niemann H. In Vitro and In Vivo Interspecies Chimera Assay Using Early Pig Embryos. Cellular Reprogramming 22:doi.org/10.1089/cell.2019.0107 (2020).
- Alowaysi M; Fiacco E; Astro V; Adamo A. Establishment of iPSC lines from a high-grade Klinefelter Syndrome patient (49-XXXXY) and two genetically matched healthy relatives (KAUSTi003-A, KAUSTi004-A, KAUSTi004-B, KAUSTi005-A, KAUSTi005-B, KAUSTi005-C). Stem Cell Res 49:102008 (2020).
- Kathuria A; Lopez-Lengowski K; Vater M; McPhie D; Cohen BM Karmacharya R. Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. Genome Medicine 12:34 (2020).
- Lee J; Bignone PA; Coles SL; Liu Y; Snyder E; Larcoca D. Induced pluripotency and spontaneous reversal of cellular aging in supercentenarian donor cells. Biochem Biophys Res Commun in press:https://doi.org/10.1016/j.bbrc.2020.02.092 (2020).
- Shimada M; Tsukada K; Kagawa N; Matsumoto Y. Reprogramming and differentiation-dependent transcriptional alteration of DNA damage response and apoptosis genes in human induced pluripotent stem cells. J Radiation Res 2019:1-10 (2019).
- Brodaczewska KK; Nielcka ZF; Maliszewska-Olejniczak K; Szxzylik C; Porta C; Bartnik W; Czarnecka AM. Metastatic renal cell carcinoma cells growing in 3D on poly‑D‑lysine or laminin present a stem‑like phenotype and drug resistance. Oncology Reports in press:2402 (2019).
- Bredenkamp N; Yang J; Clarke J; Stirparo GG; von Meyenn F; Baker D; Drummond R; Ren Y; Li D; Wu C; Rostovskaya M; Eminli-Meissner S; Smith A; Guo G. Wnt Inhibition Facilitates RNA-Mediated Reprogramming of Human Somatic Cells to Naive Pluripotency. Stem Cell Rep 13:1083 (2019).
- Nakajima M; Yoshimatsu S; Sato T; Nakamura M; Okahara J; Sasaki E; Shiozawa S; Okano H. Establishment of induced pluripotent stem cells from common marmoset fibroblasts by RNA-based reprogramming. Biochem Biophys Research Commun in press:https://doi.org/10.1016/j.bbrc.2019.05.175 (2019).
- Liu L-P; Li Y-M; Guo N-N; LI S; Ma X; Zhang Y-X; Gao Y; Huang J-L; Zheng D-X; Wang L-Y; Xu H; Hui L; Zheng Y-W. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Reports 27:455-466.e5 (2019).
- Sacco AM; Belviso I; Romano V; Carfora A; Schonauer F; Nurzynska D; Montagnani S; Di Meglio F; Castaldo C. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J Cell Mol Med :1-13; https://doi.org/10.1111/jcmm.14316 (2019).
- Nishishita N; Muramatsu M; Kawamata S. An effective freezing/thawing method for human pluripotent stem cells cultured in chemically-defined and feeder-free conditions. A J Stem Cells 4:38 (2015).
- Klein T; Klug K; Henkel L; Kwok CK; Edenhofer F; Klopocki E; Kurth I; Üceyler N. Generation of two induced pluripotent stem cell lines from skin fibroblasts of sisters carrying a c.1094C>A variation in the SCN10A gene potentially associated with small fiber neuropathy. Stem Cell Res 35:101396 (2019).
- Supharattanasitthi W; Carlsson E; Sharif U; Paraoan L. CRISPR/Cas9-mediated one step bi-allelic change of genomic DNA in iPSCs and human RPE cells in vitro with dual antibiotic selection. Sci Rep 9:174 (2019).
- Su S; Guntur AR; Nguyen DC; Fakory SS; Doucette CC; Leech C; Lotana H; Kelley M; Kohil J; Martino J; Sims-Lucas S; Liaw L; Vary C; Rosen CJ; Brown AC. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell Reports 25:3215-3228.e9 (2018).
- Ma L; Hu J; Cao Y; Xie Y; Wang H; Fan Z; Zhang C; Wang J; Wu C-T; Wang S;. Maintained Properties of Aged Dental Pulp Stem Cells for Superior Periodontal Tissue Regeneration. Aging and Disease :https://doi.org/10.14336/AD.2018.0729 (2018).
- Goa X; Yourick JJ; Sprando RL. Generation of nine induced pluripotent stem cell lines as an ethnic diversity panel. Stem Cell Researchhttps://doi.org/10.1016/j.scr.2018.07.013: (2018)
- Goa X; Yourick JJ; Sprando RI. Comparative transcriptomic analysis ofendothelial progenitor cells derived from umbilical cord blood and adult peripheral blood: Implications for the generation of induced pluripotent stem cells. Stem Cell Research25:202-212 (2017)
- Rajasingh S; Thangavel J; Czirok A; Samanta S. "Generation of functional cardiomyocytes from efficiently generated human iPSCs and a novel method for measuring contractility." PLoS one (2015)
- Poleganov MA; Eminli S; Beissert T; Herz S; Moon J-I; Goldmann J; Beyer A; Heck R; Burkhart I; Roldan DB; Tureci O; Yi K; Hamilton B; Sahin U. Efficient reprogramming of human fibroblasts and blood-derived endothelial progenitor cells using nonmodified RNA for reprogramming and immune evasion. Human Gene Therapy 26:751 (2015)
- Durruthy JD; Sebastiano V. Derivaton of GMP-compliant integration-free hiPSCs using modified mRNAs. Methods Mol Biol 1283: 31 (2014)
- Greber, B., Coulon, P., Zhang, M., Moritz, S., Frank, S., Müller-Molina, A.J., Araúzo-Bravo, M.J., Han, D.W., Pape, H.C., and Schöler, H.R. FGF signalling inhibits neural induction in human embryonic stem cells. EMBO J 30: 4874-4884(2011).
- Sugii, S., Kida, Y., Berggren, W.T., and Evans, R.M. Feeder-dependent and feeder-independent iPS cell derivation from human and mouse adipose stem cells. Nat Protoc 6(3): 346-358 (2011).
- Warren, L., Manos, P.D., Ahfeldt, T., Loh, Y.H., Li, H., Lau, F., Ebina, W., Mandal, P.K., Smith, Z.D., Meissner, A., Daley, D.Q., Brack, A.S., Collins, J.J., Cowan, C., Schlaeger, T.M., and Rossi, D.J. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7: 618-630 (2010).