Cell-based therapies have driven the regenerative-medicine wave for years. Now the focus is shifting toward cell-free alternatives, especially exosomes, which are a subtype of small extracellular vesicles (EVs). The exosomes are released by cells that carry proteins, DNA, and RNA fragments, and their contents are reflective of the cells they originate from. Because they can modulate tissue repair, inflammation, and immune responses they have become a major area of investigation in regenerative medicine research (Xu et al., 2025).
The momentum is unmistakable: more than 40,000 scientific publications now reference the term “exosomes” on PubMed (as of October 2025), illustrating how quickly interest in their therapeutic potential is accelerating. Why the rapid uptick? Exosomes bypass several limitations associated with live-cell therapies: They exhibit lower immunogenicity, do not require engraftment to exert biological effects, and can be produced, stored, and distributed in a more standardized and scalable manner. This positions exosomes as a promising modality for developing reproducible, off-the-shelf therapeutic products while reducing some of the clinical and logistical complexities inherent to cell-based approaches. Among the emerging technologies, exosomes derived from mesenchymal stromal/stem cells (MSCs), induced pluripotent stem cells (iPSCs), and the newer induced-MSC (iMSC) platforms are taking center stage. This blog provides an overview of what exosomes are, why they matter for regenerative medicine, and how iPSC-, MSC- and iMSC-derived exosome platforms compare, with an eye toward translational and manufacturing realities.
What Are Exosomes and Why They Matter
Exosomes: a subtype of EVs
EVs are membrane-bound particles secreted by virtually all eukaryotic cell types, but unlike cells, they cannot replicate. Within this broad class, exosomes originate inside the cell via an endosomal pathway: Cargo is first internalized into early endosomes, which mature into multivesicular bodies (MVBs). When the MVB fuses with the plasma membrane, its internal vesicles are released to the extracellular environment as exosomes typically smaller than 200 nm (Figure 1). In contrast, microvesicles, often confused with exosomes, are shed directly from the plasma membrane rather than via the endosomal route, a core distinction in extracellular vesicle biology.

Figure 1. Overview of exosome biogenesis and secretion. Cellular cargos are first internalized via endocytosis and sorted into early endosomes. These compartments mature through an endosomal pathway into multivesicular late endosomes (MVBs). Ultimately the MVBs merge with the plasma membrane, resulting in the release of exosomes into the extracellular space.
Role of exosomes in cell-cell communication
Exosomes are natural mediators of inter-cellular communication: the body’s own “microscopic couriers”. Exosome-based strategies leverage their natural biology to promote angiogenesis, modulate inflammation, and drive tissue repair (Saleem et al., 2024). The exosome therapeutic potential has been observed for various diseases such as inflammatory conditions, neurodegenerative and cardiovascular diseases, diabetes, wound healing, and cancer (Kalluri and Lebleu, 2020; Wang et al., 2020).
In oncology, for example, tumors exploit exosomes to remodel neighboring stromal and immune cells, promote processes such as epithelial-mesenchymal transition, invasion, metastasis, and drug resistance, and thus coordinate inter-cellular communication that amplifies malignancy. For this reason, exosomes are being investigated as biomarkers, delivery vehicles, or direct modulators of cancer progression (Dai et al., 2020).
Comparison: MSC-, iPSC- and iMSC-Derived Exosome Platforms
MSC-Derived Exosomes
MSC-derived exosomes (sometimes referred to as MSC-EVs) represent the most mature translational platform. They offer a cell-free solution with lower risk of engraftment or tumorigenicity (Zhang et al., 2018). From a translational medicine perspective, these MSC-EVs are increasingly viewed as next-generation therapeutics or delivery vehicles (Chattopadhyay et al., 2025). There are numerous preclinical animal studies confirming the therapeutic effects of MSC exosomes for stroke, Alzheimer’s disease, Type 1 diabetes mellitus, pancreatic cancer, macular degeneration, COVID-19, pulmonary infection, acute respiratory distress syndromes, and periodontitis, among others (Lofty et al., 2023).These extracellular vesicles enhance tissue repair by modulating recipient cell behavior, and delivering regenerative factors and genetic material. They also exert immunomodulatory properties by creating a pro-regenerative microenvironment and promote endogenous repair process by stimulating resident stem and progenitor cells (Roszkowski et al., 2024).
iPSC-Derived Exosomes
iPSC-derived exosomes provide one of the most scalable and engineerable avenues for extracellular vesicle-based therapies. Unlike embryonic stem cells, iPSCs are readily obtainable with minimal ethical concerns, support master-cell banking, and allow consistent large-scale expansion. They are particularly well suited for advanced cargo (engineered payloads) or surface modifications, making them an attractive platform for companies pursuing customizable exosome content, complex therapeutic indications, or sophisticated delivery strategies. iPSC-derived extracellular vesicles therefore offer unmatched flexibility and innovation potential.
Recent studies show that iPSC-derived exosomes carry powerful regenerative capabilities. iPSC exosomes have demonstrated to have superior regenerative performance in corneal epithelial repair compared to MSC-derived exosomes. In a 2020 study (Wang et al., 2020), iPSC exosomes accelerated wound closure and promoted epithelial cell proliferation and migration more effectively, indicating a more potent pro-healing biological profile in this tissue context.
For fibroblasts, they lower senescence markers such as β-galactosidase and help sustain collagen production following UV damage (Oh et al., 2018). In vascular cells exposed to high-sugar conditions, they preserve cell viability and structural integrity (Ding et al., 2018). When applied to stem cells, including MSCs and iPSCs, they restore proliferative capacity, in several studies, outperform exosomes derived from MSCs (Kim et al., 2018; Liu et al., 2019; Wang et al., 2020). Their therapeutic activity has now been reported across multiple tissue systems involving the heart, limb, liver, skin, bone, eye, and neurological models (Wang et al., 2021). Collectively, these findings highlight iPSC exosomes as a promising cell-free therapeutic tool for enhancing tissue repair and regeneration.
iMSC-Derived Exosomes
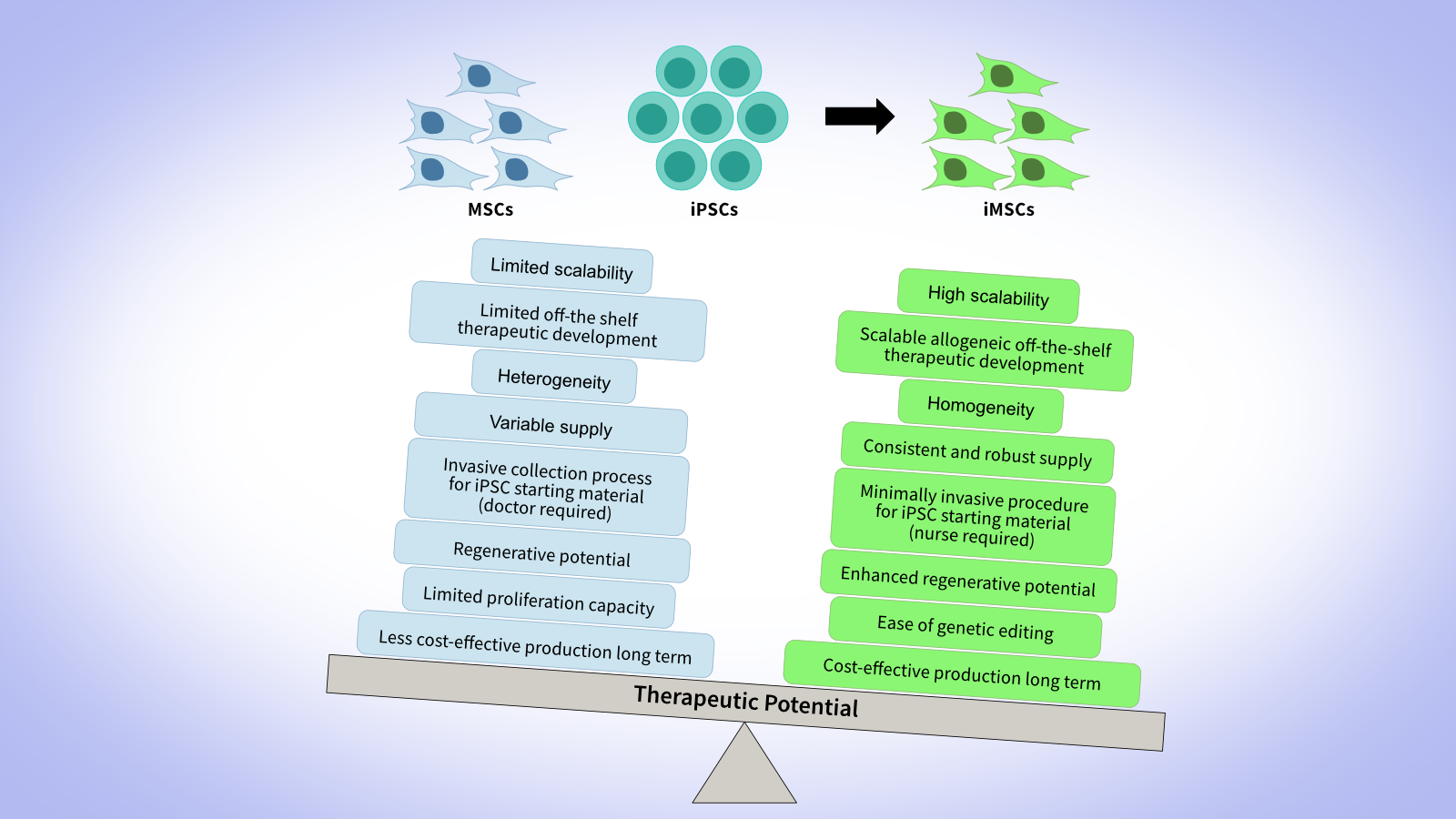
iMSCs are generated by differentiating iPSCs in vitro, allowing essentially unlimited propagation and providing a consistent source of starting material. This approach enables exosomes derived from iMSCs to combine the manufacturing scalability, homogeneity, robustness and reproducibility of iPSC-based processes with the familiar mesenchymal phenotype observed in MSCs, offering a steady supply for clinical and research use.
Although large-scale master and working cell bank (MCB and WCB) production of iMSCs is recognized to become more cost-effective with scale compared to standard MSC cell banks, it remains to be shown whether this translates to exosomes derived from those cell types. iMSCs yield exosomes that mimic the regenerative and immunomodulatory properties of primary MSC exosomes, and in some cases may outperform tissue-derived MSC exosomes (Sabapathy and Kumar, 2016). For example, one study found that exosomes from iMSCs achieved greater therapeutic effect in an osteoarthritis model than synovial membrane MSC-exosomes (Zhu et al., 2017). For translational scientists and process engineers, iMSCs offer a pathway to standardized, scalable exosome production, helping mitigate donor variability and supply-chain complexity.
Clinical & Translational Landscape:
MSC, iPSC and iMSC-Derived Exosome Therapies
Exosomes derived from MSCs, iPSCs and iMSCs have demonstrated robust efficacy in animal models of tissue injury - including lung, muscle, cartilage, skin, and neural repair. In humans, early clinical trials delivering MSC-EVs via topical, intra-articular or inhalation routes have reported acceptable safety and encouraging “signals” such as faster wound healing and improved lung function (Han et al., 2025).
Clinical exploration of iPSC-derived exosomes is also expanding, with ongoing trials assessing their use in stable vitiligo (NCT06810869), atopic dermatitis (NCT05969717), and Moyamoya disease (NCT07065409). In contrast, clinical studies of iMSC-derived exosomes remain largely absent, highlighting the early and exploratory stage of their translational development.
Despite this growing momentum, several key barriers continue to limit clinical translation. Scalable, high-yield production of exosomes with consistent cargo composition and bioactivity remains technically challenging. The lack of standardized potency assays, biodistribution studies, and dosing metrics further complicates regulatory evaluation and cross-study comparison. Additionally, rapid in vivo clearance, variability introduced by donor cell state and culture conditions, and limited data on long-term stability and storage all pose ongoing hurdles. Most current clinical studies are small and non-randomized, and they employ heterogeneous potency endpoints—underscoring the urgent need for harmonized methodologies and larger, controlled trials. Nevertheless, for stakeholders across biotechnology, academia, and investment, exosome therapeutics derived from MSCs, iPSCs, or iMSCs represent a compelling “off-the-shelf,” cell-free regenerative strategy. Success will depend on overcoming manufacturing, standardization, and regulatory challenges that currently define this rapidly evolving field.
Choosing the Right Exosome Cell Source
Selecting an optimal exosome source - MSC, iPSC, or iMSC - requires balancing biological, translational, and manufacturing considerations (Table 1). MSCs offer advantages of regulatory familiarity and established clinical workflows, enabling faster clinical translation. However, they may present challenges in scalability and genetic engineering. In contrast, iPSCs and iMSCs provide a renewable, highly scalable platform capable of producing consistent, customizable cell banks. These advantages come at the cost of more complex process development, validation, and regulatory oversight.
| Feature | MSC-Derived Exosomes | iPSC-Derived Exosomes | iMSC-Derived Exosomes |
|---|---|---|---|
| Cell Source |
Adult tissue-derived MSCs (bone marrow, adipose tissue, umbilical cord) |
iPSCs (reprogrammed somatic cells) |
MSC-like cells differentiated from iPSCs (iPSC → MSC transition) |
| Scalability & Supply |
|
|
|
| Engineering & Customization |
|
|
|
| Translational Familiarity & Clinical Precedent |
|
|
|
| Risk Profile & Regulatory Considerations |
|
|
|
| Batch & Donor Variability |
|
|
|
| Therapeutic Evidence & Domain Strengths |
|
|
|
| Key Manufacturing & CMC Considerations | Platform more mature, but still requires rigorous upstream& downstream control | Demands development of iPSC MCBs, purification & dosing strategies | Needs process development for differentiation (iPSC → iMSC), standardization and scale-up of exosome output |
| Strategic Use-case Recommendation |
Ideal for proof-of-concept, faster time to clinic, familiar regulatory pathway | Ideal for long-term engineered therapies, modular cargo, highly customized applications | Ideal for scalable, off-the-shelf standardized exosome products with MSC phenotype and engineered potential |
Table 1. Comparison: MSC- vs iPSC- vs iMSC-Exosome therapeutic platforms
Manufacturing & Quality:
Turning Exosome Biology into Therapeutics
Manufacturing and quality control are the pivotal link between early research and clinically viable exosome therapeutics. While traditional flask-based culture and ultracentrifugation techniques may suffice for the discovery stage, they are inadequate for GMP-grade production: where scalability, reproducibility and batch-to-batch consistency become major obstacles.
To meet therapeutic standards, a transition to closed bioreactors and industrial downstream purification using filters or membranes must replace open-system workflows to achieve controlled, contamination-resistant, and traceable production. Additionally, rigorous analytics are required to characterize exosomal preparations, measuring particle size, concentration, surface markers, cargo and stability to ensure each batch meets defined identity, purity and potency criteria. Given that extracellular vesicles exhibit inherent heterogeneity and are highly sensitive to donor cell state and process conditions, developing standardized manufacturing workflows and well-defined release specifications (akin to those adopted in viral-vector production) is essential. Only by treating exosome production with the same rigor as biologics manufacturing can the promise of exosomes be reliably translated into safe, reproducible therapeutic products (Zhang and Cheng, 2023).
Key Take-aways
- Exosomes are a cell-free alternative to whole-cell therapies, but the term demands precision (e.g. size, origin, cargo, biogenesis).
- MSC-derived exosomes remain the most advanced in development, but iPSC and iMSC platforms bring scalability and engineering potential.
- Manufacturing and quality control are essential; without them, reproducibility and regulatory viability crumble.
- Early human data are encouraging, but standardization of potency, assays, and source material remains a bottleneck.
How REPROCELL Can Support Your Exosome Therapeutics Journey
If you are developing exosome-based therapies, REPROCELL offers an integrated suite of capabilities spanning the full value chain - from donor-sourced material and cell-banking, through manufacturing, analytics and translational support. We deliver milestone-based, customized programs:
from RNA-based iPSC reprogramming to GMP-compliant MCB manufacturing, engineered iMSC lines, downstream purification and characterization platforms tailored for EVs.
Explore how we can help for your exosome projects:
- REPROCELL's iPSC-derived Exosomes
- REPROCELL Japan GMP Manufacturing Facility
- Understanding iPSC-derived Exosomes
- FAQ: iPSC Exosomes - Preclinical Research Overview
- Production & characterization of cell-derived biologics
References
- Xu G; Jin J; Fu Z; et al. Extracellular vesicle-based drug overview: research landscape, quality control and nonclinical evaluation strategies. Sig Transduct Target Ther. 10 (2025).
- Saleem M; Shahzad K.A; Marryum M; et al. Exosome-based therapies for inflammatory disorders: a review of recent advances. Stem Cell Res. Ther. 15 (2024).
- Kalluri R; LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science 367 (2020).
- Wang S; Hou Y; Li X; et al. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging (Albany NY) 12:19546–19562 (2020).
- Dai J; Su Y; Zhong S; et al. Exosomes: key players in cancer and potential therapeutic strategy. Transduct. Target Ther. 5:145 (2020).
- Zhang S; Jiunn S.C; Lai R.C; et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156 (2018).
- Chattopadhyay S; Rajendran R.L; Chatterjee G; et al. Mesenchymal stem cell-derived exosomes: a paradigm shift in clinical therapeutics. Experimental Cell Research 450:114616 (2025).
- Lofty A; AboQuella NM; Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Research & Therapy 14, 66 (2023).
- Roszkowski S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clinical and Experimental Medicine 24(1):46 (2024).
- Oh M; Lee J; Kim Y.J; Rhee W.J; Park J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. International Journal of Molecular Sciences. 19:1715 (2018).
- Ding Q; Sun R; Wang P; et al. Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Experimental and therapeutic medicine. 15:4791–4797 (2018).
- Kim S; Lee S.K; Kim H; et al. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. International Journal of Molecular Sciences 19 (2018).
- Liu S; Mahairaki V; Bai H; et al. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 37:779–790 (2019).
- Wang A.Y.L. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. International Journal of Molecular Sciences 22(4):1769 (2021).
- Sabapathy V; Kumar S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. Journal of Cellular and Molecular Medicine 20(6) 1571-1588 (2016).
- Zhu Y; Wang Y; Zhao B; et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research & Therapy 8, 64 (2017).
- Han X; Liao R; Li X; et al. Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies. Signal Transduction and Targeted Therapy 10:262 (2025).
- Induced Pluripotent Stem Cell Derived Exosomes Nasal Drops for the Treatment of Stable Vitiligo (NCT06810869). gov (National Library of Medicine). Retrieved November 12, 2025.
- Induced Pluripotent Stem Cell Derived Exosomes for the Treatment of Atopic Dermatitis (NCT05969717). gov (National Library of Medicine). Retrieved November 12, 2025.
- Treatment of Moyamoya Disease With iPSC-derived Exosomes (NCT07065409). gov (National Library of Medicine). Retrieved November 12, 2025.
- Abbasi R; Alamdari-Mahd G; Maleki-Kakelar H; et al. Recent advances in the application of engineered exosomes from mesenchymal stem cells for regenerative medicine. European Journal of Pharmacology 989 (2025).
- Malik S; Muhilan Y; Nordin F; et al. Stem cell derived exosome trilogy: an epic comparison of human MSCs, ESCs and iPSCs. Stem Cell Research & Therapy 16, 318 (2025).
- Zhang K; Cheng K. Stem cell-derived exosome versus stem cell therapy. Nature Reviews Bioengineering 1, 608–609 (2023).



.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



