How to Characterize the Barrier Properties of Alvetex®-Based Mucosal Models
Mucosal tissues, such as intestine and skin, are crucial mediators of homeostasis as they function to protect the internal host tissues from the external environment by forming a barrier impenetrable to microorganisms. The gold-standard model of an intestinal barrier is a monolayer of epithelial Caco-2 cells. These cells are typically cultured for 21 days as a monolayer on Transwell® polycarbonate inserts and are used for drug permeability studies and are essential in characterizing the safety and efficacy of new chemical entities before they are used in animal and human trials [1,2].

REPROCELL’s contract research services use human living tissues to better predict drug safety and efficacy.
However, Transwell models have a number of limitations including the lack of communication between multiple cell types and alterations in protein expression and activity to the in vivo human intestine. As such, more complex models, that encompass multiple cell types, have been developed to overcome some of the Caco-2 monolayer limitations. In this post we will provide an insight into how to properly characterize the barrier structure and function of a novel model of the intestinal mucosa developed using 12-well Alvetex® scaffold as a platform for cell growth.
.png?width=809&name=Schematic%20protocol%20detailing%20the%20development%20of%20an%20intestinal%20mucosal%20model%20(A).png)
.png?width=321&name=Schematic%20protocol%20detailing%20the%20development%20of%20an%20intestinal%20mucosal%20model%20(B).png)
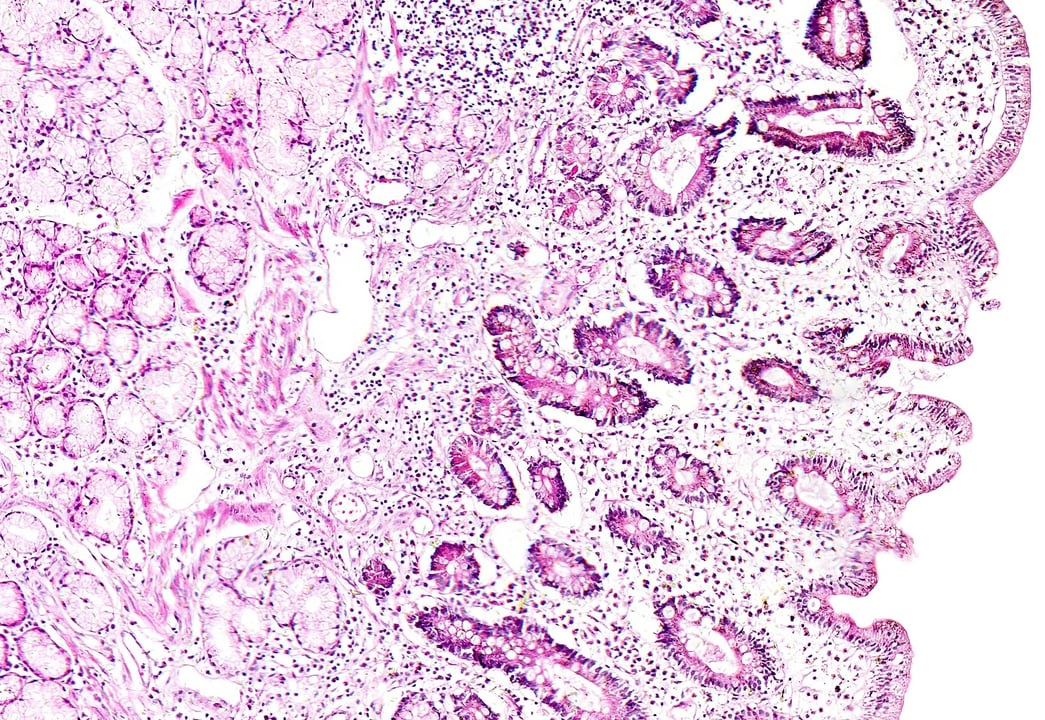
Fig 1. Schematic protocol detailing the development of an intestinal mucosal model. Fibroblasts are seeded into Alvetex® Scaffold inserts to resemble a lamina propria. Caco-2 cells are seeded on the surface of the fibroblasts and maintained for 21 days to ensure Caco-2 differentiation before analysis of models (A). H&E of the Caco-2/fibroblast co-culture; Caco-2 cells display a highly polarised morphology when cultured on the surface of fibroblasts. Scale bar: 20 µm (B).
How to Examine Barrier Quality by Transport Assays
The structural integrity of the mucosal model barrier can be determined through transport assays of hydrophilic integrity markers such as the fluorescent marker lucifer yellow [3]. These assays can be done in combination with transport assays for other non-fluorescent drug compounds such as atenolol or digoxin once the mucosal models have fully differentiated. Transport assays can be performed in either the apical-basolateral direction (described herein) or the basolateral-apical direction and are typically performed in a 12-well plate for ease of use.
- If using the 12-well Alvetex® format, snap off the arms of the plastic insert and wash models in Hanks Buffered Saline Solution (HBSS) for a minimum of 15 minutes at 37°C.
- Add 1.5 mL HBSS to each well of a fresh 12-well plate and transfer models to this fresh buffer solution. The buffer should only be in contact with the basolateral surface.
- Dilute lucifer yellow to 100 µM in HBSS and add 400 µL solution to the apical surface of the insert.

Fig 2. Apical-basolateral transport of lucifer yellow transport assay used to determine the barrier integrity of the model.
- Incubate on an orbital shaker at 50 rpm at 37°C.
- At selected timepoints remove 50-100 µL buffer from the receiver, basolateral compartment and replace with fresh buffer solution.
- Once the transport assay has finished, calculate the apparent permeability coefficient or the percentage rejection of lucifer yellow using the following equations:
Where dQ/dT is the rate of transport of a compound, Vr is the volume of the receiver compartment, C0 is the initial compound concentration at T = 0 and A is the surface area (cm2).
Lucifer yellow rejection of over 90 % is considered to be a strong barrier.
Determination of Barrier Quality by Immunostaining
Whole-mount immunostaining techniques can be used to check that the epithelial layer is forming the appropriate junctional complexes in the proper anatomical locations. For example, tight junction and adherens junction markers should be expressed at the apical surface of epithelial cells and should surround the entirety of the cell creating a cobblestone morphology typical of an epithelium layer.
- Unclip models from Alvetex® inserts and place into 12-well plates, ensuring that the apical surface of the model is facing upwards.
- Wash each sample 3× in PBS and fix in a 1:1 solution of ice-cold methanol:acetone for 20 minutes at -20°C.
- Wash samples 3× in PBS and add 0.5 mL blocking buffer/well and incubate at room temperature for 1 hour.
- Dilute primary antibodies, specific to components of junctional complexes, in blocking buffer according to manufacturer’s instructions.
- On parafilm, pipette out 100 µL of primary antibody and place the mucosal model apical side down on the antibody solution. Incubate for 1-2 hours at room temperature.
- Transfer the samples back to the 12-well plate, again ensuring that the apical surface of the model is facing upwards and wash 3× in PBS for 10 minutes.
- Dilute secondary antibodies that are conjugated to fluorophores in blocking buffer according to manufacturer’s instructions and as before, pipette 100 µL antibody solution onto a clean piece of parafilm.
- Place the models on top of the antibody solution, apical side down and incubate in the dark for 1 hour at room temperature.
- Wash samples 3 × 10 minutes in PBS in a 12-well plate.
- To mount samples, place a drop of Vectashield/DAPI hardset onto a coverslip and transfer the Alvetex® onto the solution basolateral side down.
- Place a further drop of Vectashield/DAPI hardset on the surface of the Alvetex® model and seal by adding a coverslip on top.
Image samples using a Z-stack imaging set-up on a confocal microscope.
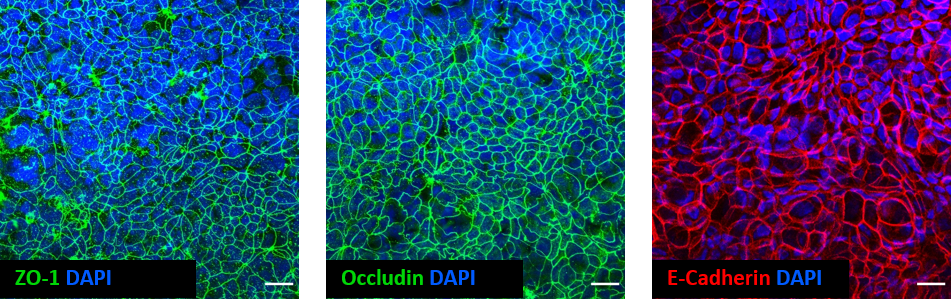
Fig 3. Whole-mount immunostaining of a Caco-2 fibroblast co-culture model of the intestinal mucosa created using 12-well Alvetex Scaffold as a platform for cell culture. Scale bar: 20 µm.
Complete in-depth characterization
Before using mucosal models within industry and clinical trials, it is important to ensure that an in-depth characterization of the models’ structure and function has been performed. Two key methods of characterization have been described above, and these along with other techniques such as electron microscopy, should be utilized to ensure that in vitro cultures anatomically and functionally represent the in vivo tissue being studied.
Meet the inventor of Alvetex: Video Interview with Prof Stefan Przyborski

REPROCELL’s contract research services use human living tissues to better predict drug safety and efficacy.
References
- Hidalgo et al. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96(3):736–49 (1989)
- Artursson et al. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 1;46(1-3):27–43 (2001)
- Hubatsch et al. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2(9):2111–9 (2007)

Author
Nicole Darling
Subscribe to receive updates from REPROCELL
Tagged
Stem cell and drug discovery scientists around the world are using REPROCELL’s services and products in their preclinical and clinical research.