REPROCELL’s Growth Strategy
What are iPS Cells?
Induced pluripotent stem (iPS) cells are so-called “pluripotent cells” created by introducing specific factors into somatic cells, such as skin or blood cells, reprogramming them to a state close to that of fertilized eggs. iPS cells were developed in 2006 by Professor Shinya Yamanaka of Kyoto University (recipient of the 2012 Nobel Prize in Physiology or Medicine) and his colleagues.
iPS cells possess two defining characteristics: the ability to proliferate almost indefinitely, and pluripotency – the capacity to differentiate into virtually any cell type in the human body, including nerve cells, cardiomyocytes, and blood cells. Because of these properties, iPS cells are expected worldwide to play a major role in regenerative medicine, which aims to restore functions lost due to disease or injury, as well as in drug discovery, where patient-derived cells are used to elucidate disease mechanisms and develop new therapeutics.
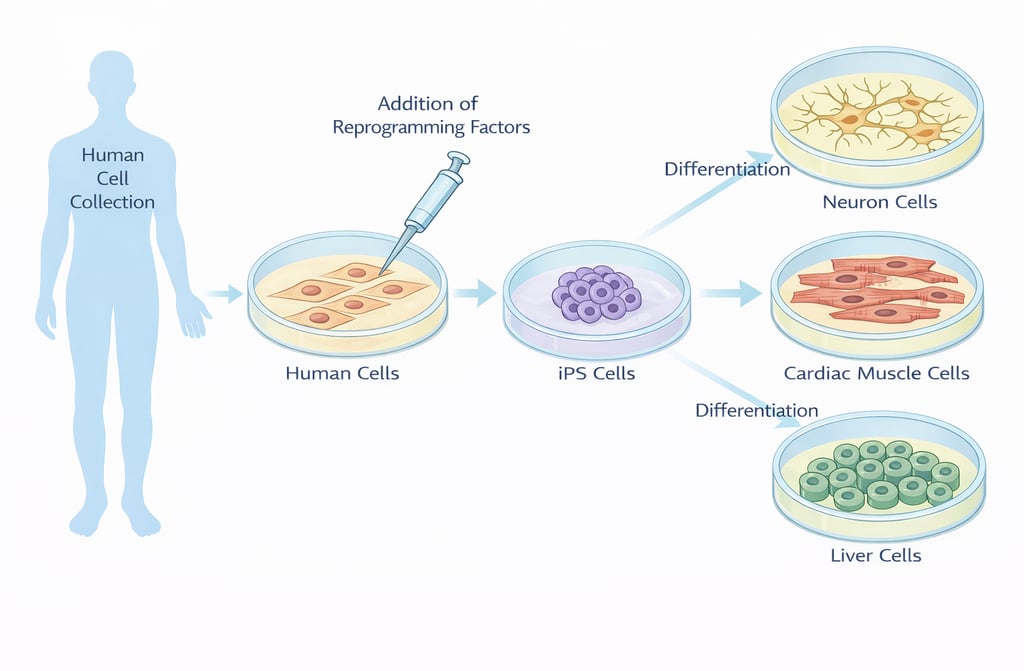
REPROCELL, Inc. is a company that has grown alongside this groundbreaking iPS cell technology. As a “regenerative medicine platform company centered on iPS cell technology,” we continue to take on the challenge of establishing new treatments for intractable diseases and cancer.
Our growth strategy is supported by three pillars built on the iPS cell technology platform:
- Development of regenerative medicine products (pipeline) that drive mid- to long-term growth
- Clinical-grade cell manufacturing infrastructure (CDMO) that supports treatments with global-standard quality
- Research support products and services that generate stable revenue
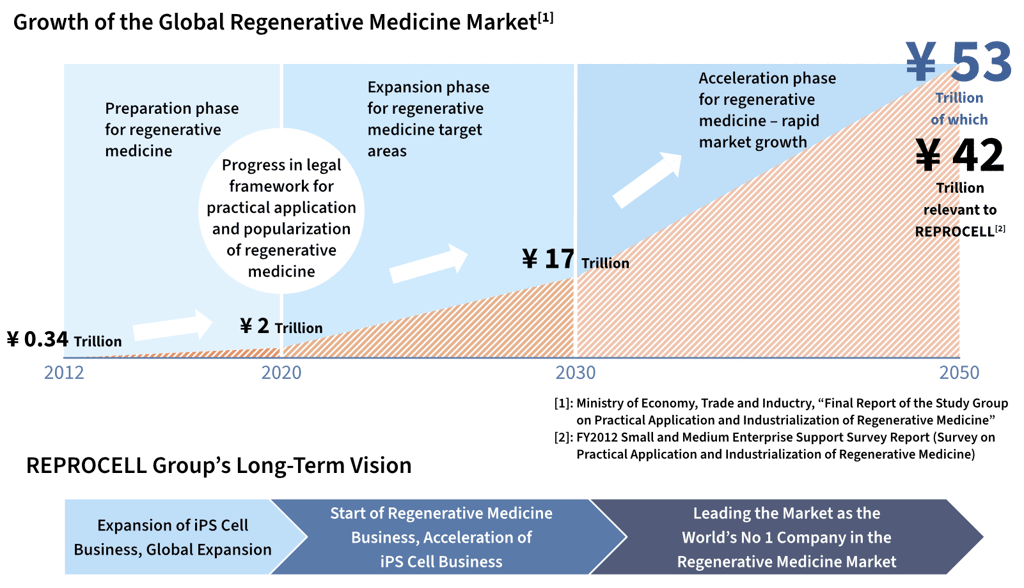
Through the close integration of these three business domains, we aim to become the world’s No.1 company in the regenerative medicine market, which is expected to expand significantly toward 2030 and 2050.
1. Four Regenerative Medicine Pipelines Driving Mid- to Long-Term Growth
We are currently advancing the development of four major regenerative medicine products in the fields of neurological disorders and cancer, where effective treatments have yet to be established. The commercialization of these products is expected to drive substantial business growth.
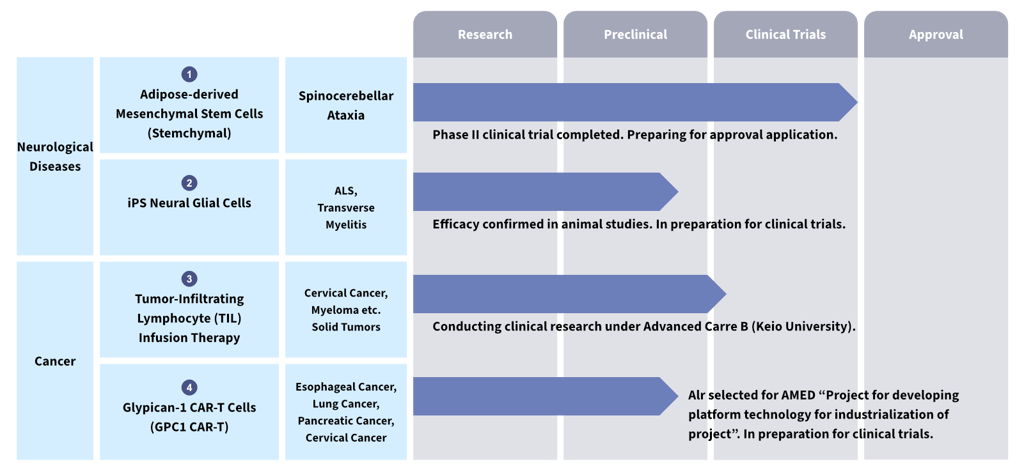
Stemchymal for Spinocerebellar Ataxia
Spinocerebellar ataxia is a progressive intractable disease characterized by gradual loss of motor function, ultimately leading to a bedridden state. At present, there are no effective drugs that can suppress disease progression. Stemchymal (adipose-derived mesenchymal stem cells), developed by ReproCELL, has completed Phase II clinical trials in Japan and Taiwan. Data obtained suggest improvements in the SARA score (an index for evaluating ataxia) and long-term stabilization of symptoms. We are currently preparing regulatory approval applications in both Japan and Taiwan, aiming for early commercialization.
iPS-Derived Neural Glial Cells for ALS
For amyotrophic lateral sclerosis (ALS), a designated intractable disease with rapid progression and no curative treatment, we are developing a therapy using iPS cell–derived neural glial cells. In animal studies, suppression of motor function decline and maintenance of motor neuron survival have been confirmed. We are now preparing for clinical trials, while also considering future expansion to other neurodegenerative diseases such as transverse myelitis.
Tumor-Infiltrating Lymphocyte (TIL) Therapy for Solid Tumors
TIL therapy involves isolating and culturing lymphocytes from a patient’s own tumor tissue to enable them to attack cancer cells, and is attracting attention as a new treatment option for solid tumors such as cervical cancer and melanoma. In the United States, FDA approvals have already been granted, increasing global interest in this therapy. ReproCELL is responsible for cell manufacturing in Keio University’s Advanced Medical Care B program for TIL therapy targeting cervical cancer, with actual patient administration having commenced in November 2024.
Next-Generation Immunotherapy: Glypican-1 (GPC1) CAR-T Cell Therapy
While conventional CAR-T therapies have shown high efficacy in hematological malignancies, challenges remain in their application to solid tumors. ReproCELL is developing a next-generation CAR-T therapy targeting Glypican-1 (GPC1), which is specifically expressed in solid tumors such as squamous cell carcinoma and pancreatic cancer. This project has been selected for support by AMED (Japan Agency for Medical Research and Development), and we are accelerating preparations for clinical trials through joint development with academic institutions.
2. Clinical-Grade Cell Manufacturing Infrastructure and Proprietary Technologies Across Japan, the USA, and Europe
High-quality and safe cell manufacturing technology is essential for the commercialization of regenerative medicine. ReproCELL operates GMP-compliant cell processing facilities in three regions—Japan, the United States, and Europe (Spain through a partner company)—and has established a global manufacturing system that complies with regional regulations (PMDA, FDA, EMA). This enables us to provide end-to-end manufacturing services, from tissue procurement to final product manufacturing.
Our technological competitive advantages lie in safety and versatility.
mRNA Reprogramming Technology
By using mRNA instead of viral vectors, we can generate iPS cells without damaging the genome. This significantly reduces the risks of tumorigenesis and genetic abnormalities, ensuring extremely high safety for clinical applications.
HLA Knockout Technology
Through proprietary genome-editing technology, we have successfully created iPS cells in which HLA (human leukocyte antigen), a major cause of immune rejection, has been eliminated. This innovative technology forms the basis for “universal donor cells” that can be transplanted into any patient.

 Tonomachi REPROCELL Regenerative Medicine Center
Tonomachi REPROCELL Regenerative Medicine Center
- Certification: Authorized for Manufacture of Specified (Processed) (Cellular) Products
- Regulations: PMDA
- Role: Domestic Development and Manufacturing of Regenerative Medicine Products

 GMP Cell Processing Facility (Maryland, USA)
GMP Cell Processing Facility (Maryland, USA)
- Certification: GMP Compliant
- Regulations: FDA
- Role: Global Clinical Trials and CDMO Manufacturing
In addition, we are promoting research and development of “iPS exosomes,” which are highly purified and concentrated extracts derived from iPS cell culture supernatants. By leveraging their effects on cellular rejuvenation and maintenance of collagen production, we aim to expand into new medical and healthcare fields.
3. Global Research Support Platform Supporting Drug Discovery and Research Worldwide
Leveraging the technological expertise accumulated since our founding, we provide products and services that support cutting-edge research conducted by academic institutions and pharmaceutical companies worldwide. Through our four global bases in Japan, the United States, the United Kingdom, and India, we deploy business operations tailored to regional needs.
Our business portfolio includes reagent sales in the U.S. market, pharmacology and efficacy testing using human tissues in Europe, and genome analysis services in India, making full use of regional strengths.
By serving as a stable revenue base, these research support services enable long-term investment in regenerative medicine product development, allowing us to continue taking on challenges while maintaining a sound and sustainable management structure.
Research Drugs and Cell Products
Research Contract Services

Future Outlook
The regenerative medicine market is entering a phase of rapid expansion alongside advances in regulatory frameworks, and is projected to reach a global scale of approximately 53 trillion yen by 2050.
By securing stable revenue through research support services while achieving multiple approvals and commercializations of regenerative medicine products, ReproCELL aims to realize non-linear growth. Through delivering innovative therapies to patients around the world, we will continue to pioneer the future of regenerative medicine.