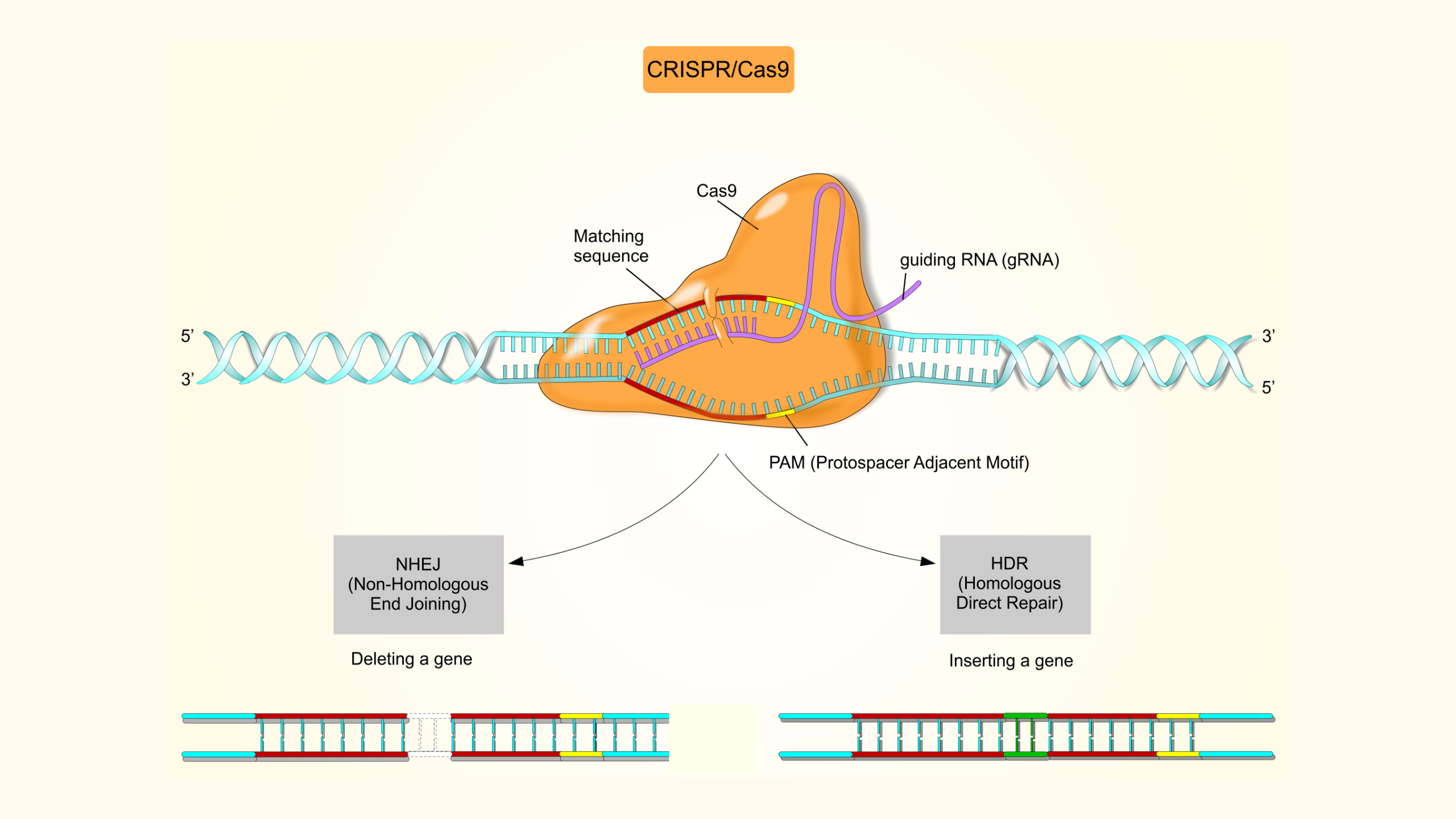
StemEdit is REPROCELL’s advanced genome-editing service, tailored specifically for iPSCs. Whether your goal is to support cutting-edge research or generate gene-edited iPSC lines for clinical applications, our expert team provides high-precision gene modifications designed to meet your exact project requirements.
REPROCELL enables you to obtain the precise genotype required, saving time and resources ahead of large-scale cell bank production. You may provide your own iPSCs or select from our research- and clinical-grade iPSC clones.
Alternatively, we can generate custom iPSC lines, tailored to your specific project goals. Contact us for more details.
High-Precision Genome Editing for Stem Cells
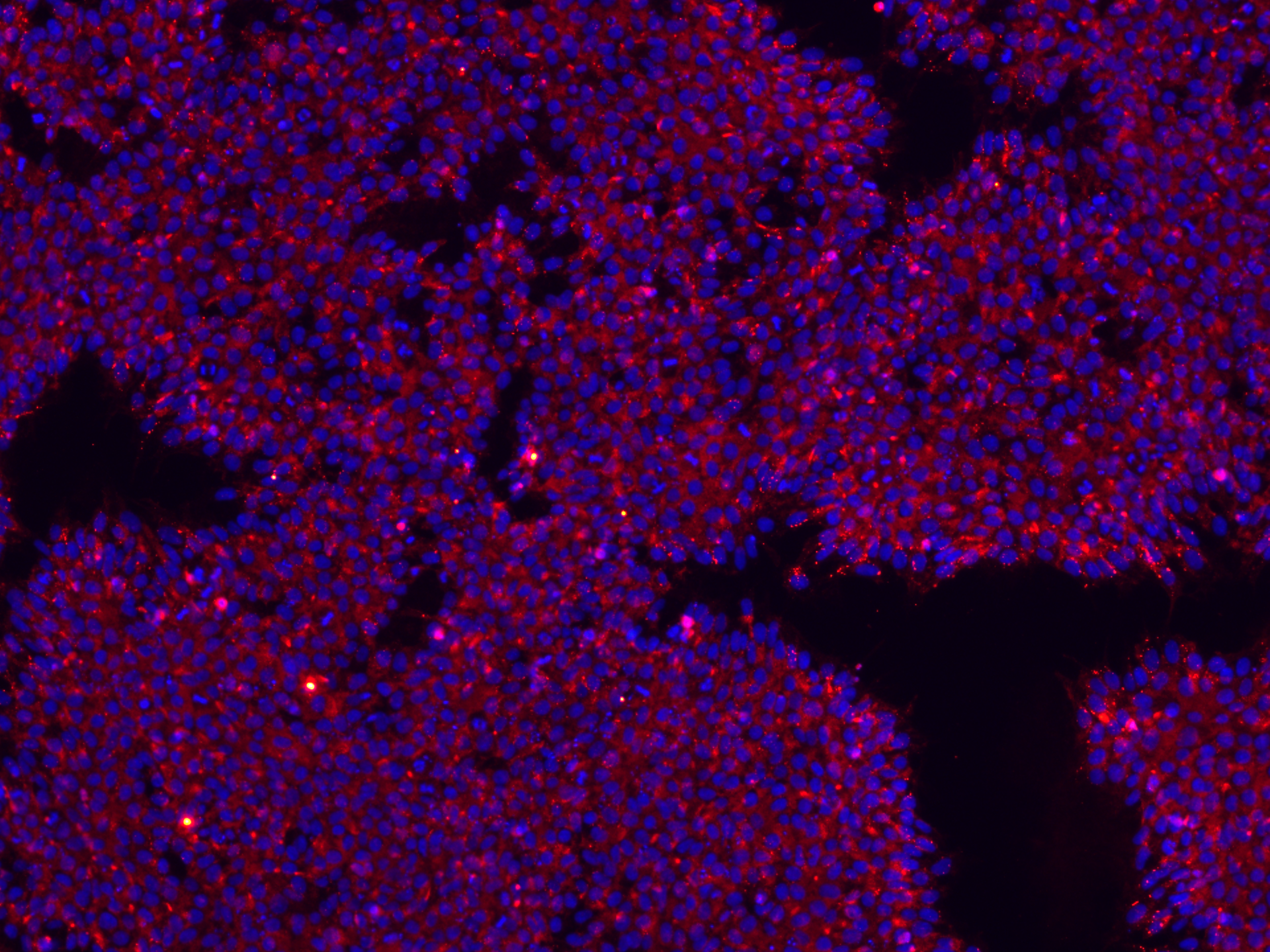
The StemEdit service delivers high-precision gene editing in stem cells, enabling complex modifications early in your project development. Optimized for a variety of nuclease systems (including caspases (Cas enzymes)), StemEdit achieves challenging edits reliably, helping to reduce risk and accelerate progression to downstream cell bank manufacturing.
Examples of genetic modifications include:
- Knock-in of large gene fragments
- Knock-in of biallelic mutations
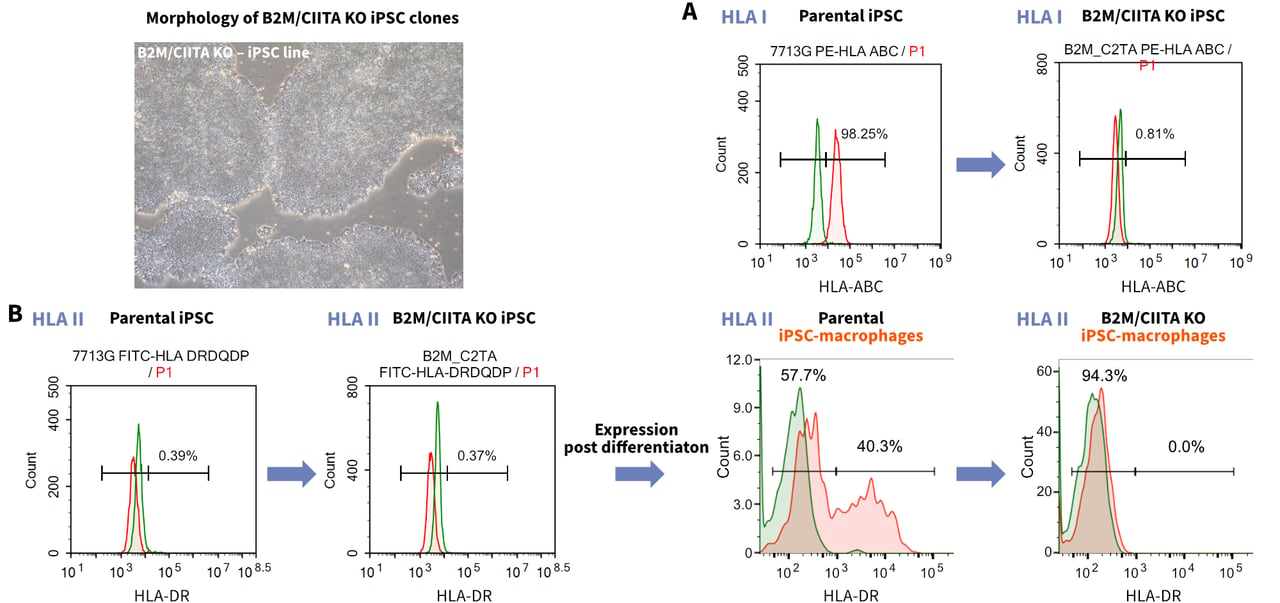
- Knock-out of multiple genes
- Incorporation of reporter or selection genes
Global StemEdit Facilities: Gene Editing in USA & Japan
StemEdit projects can be carried out at either our REPROCELL USA facility in Beltsville, Maryland, or at our REPROCELL Japan GMP Manufacturing Facility, depending on your project’s scope and logistical needs. Choose the location that best matches your timelines, regulatory requirements, and desired throughput.
REPROCELL Stemgent™ – the stem cell experts
Our broad range of products and services for stem cell scientists are used by leading pharmaceutical and biotechnology companies as well as top academic and government research institutions all around the globe.
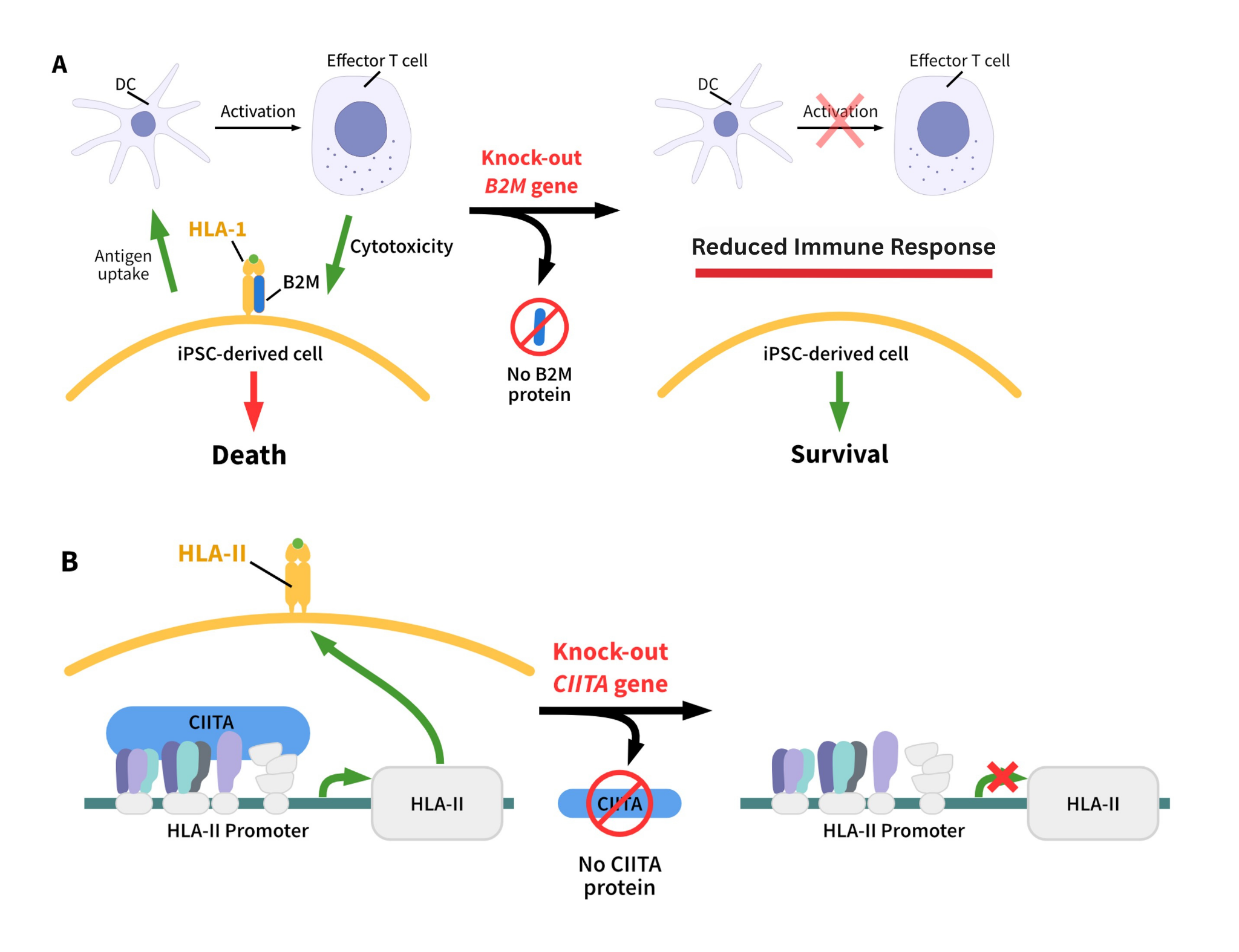

.jpeg)