- Product Catalog
- Featured
- StemEdit™ Gene Edited iPSCs
StemEdit Gene Edited Research iPSCs
Off-the-shelf hypoimmune cell lines
REPROCELL has developed high-quality HLA knockout iPSC lines using StemEdit—our state-of-the-art genome editing technology, which takes advantage of an AI-enhanced nuclease system. It is also well-suited for clinical gene-editing projects.
New Offer
The clinical parental control iPSC line—used to develop our two StemEdit hypoimmune iPSC lines—is now available in an affordable bundle with the gene-edited clones.
Interested? Reach out directly at info-us@reprocellusa.com for details.
We offer the following products to meet the research needs of our customers:
StemEdit Human iPSC non-HLA class 1/2
(B2M/CIITA Homo double KO)
B2M/CIITA Double Knockout iPSC Line:
StemEdit hiPSC B2M/CIITA DKO RPC-LLF-34-F3
suppresses both HLA class 1 and HLA class 2 expression

StemEdit hiPSC B2M/CIITA DKO RPC-LLF-34-F3
(B2M/CIITA Homo double KO)
(cat. no. RCRP052)
Benefits of StemEdit human iPSCs
By using our cell lines, you can immediately start your research using HLA knockout iPSCs without the hassle of editing iPSC lines yourself. In both cell lines, either one or both alleles (homozygous) of the target genes (B2M, CIITA) are knocked out, and this reliably suppresses HLA expression.
✓
High-quality genome editing: Powered by highly efficient StemEdit technology, which is distinct from CRISPR/Cas9.
✓
Homozygous knockout: Mutations are introduced into both alleles of the target gene to ensure reliable suppression of expression.
✓
License: These cells are intended for Research Use Only (RUO), and no additional license is required for basic research purposes, including animal testing. If you are considering commercial use and/or clinical applications of edited iPSC lines, REPROCELL can providesolutions for this as well. Please contact us for further details.
✓
Clinical-grade derivation: REPROCELL's StemRNA high-quality clinical-grade iPSC lines are used as the parent strains.
The introduction of the new hypoimmune StemEdit Human iPSC lines marks a significant milestone in advancing immune research and regenerative medicine. By enabling researchers to study and manipulate immune cell interactions with unprecedented precision, these cell lines exemplify REPROCELL's commitment to empowering scientific discovery. We will continue to innovate and provide world-class tools to support breakthroughs in cell therapy and immunology.
— Dr. Chikafumi Yokoyama, CEO of REPROCELL Inc.
The Science of StemEdit human iPSCs
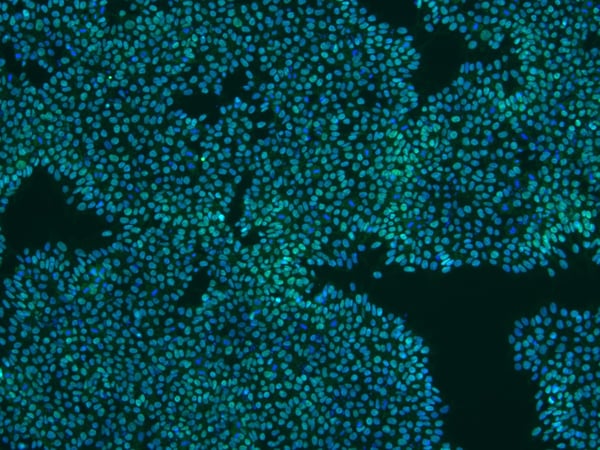
StemEdit human iPSCs grown in vitro stain positive for standard pluripotency markers
StemEdit Human iPSC non-HLA class 1 (B2M Homo KO) and non-HLA class 1/2 (B2M/CIITA Homo double KO) cells were cultured using NutriStem hPSC XF Medium (01-0005) on iMatrix-511 (NP892-011). Pluripotency for both cell lines was confirmed by the strong expression of Nanog, Oct3/4, and SSEA4, as well as negative for the negative marker SSEA-1.
Below: B2M/CIITA Homo double KO cells.

StemEdit™human iPSCs exhibit the expected karyotype
Karyotyping of StemEdit Human iPSC non-HLA class 1/2 (B2M/CIITA Homo double KO)(RCRP052) was performed, and the strain displayed a normal karyotype (46, XX).

Confirmation of HLA class 1 expression suppression
HLA class 1 expression levels in both the double knockout strain and the parent strain (LLF-34-F3) were evaluated by flow cytometry analysis using an anti-HLA-ABC antibody. HLA class I expression was confirmed in the parent strain, and HLA class I expression was significantly suppressed in B2M/CIITA double KO (RCRP052), where the B2M gene was knocked out.

StemEdit™ Human Hypoimmune iPSCs
| Cat. No. | Strain ID | Donor Race | Donor Sex | Donor Age | Donor Blood Type | Donor Clinical Status |
Reprogramming |
Gene Editing Technology | Tissue Source |
| RCRP052 | StemEdit hiPSC B2M/CIITA DKO RPC-LLF-34-F3 |
Caucasian | Female | 22 | O positive | Healthy | StemRNA™ Clinical Reprogramming Technology
|
StemEdit OpenCrispr-1-based gene editing
|
Fibroblasts
|
High-quality parent line: Clinical-grade iPSC line LLF-34-F3
Pioneering iPSC-based Therapies with Clinical-Grade iPSCs
The StemRNA Clinical-grade iPSC line LLF-34-F3, established by REPROCELL, is used as the parent line for the preparation of the StemEdit HLA knockout iPSC lines. Our clinical-grade iPSCs have the following features.
- Regulatory compliance: Developed and manufactured in a process complying with the FDA (United States), EMA (Europe), and PMDA (Japan) regulatory requirements.
- Rigorous Donor Screening: Healthy donors who have undergone appropriate screening following regulatory guidelines with ethical considerations.
- Manufacturing at a GMP-compliant facility: Manufactured and frozen under strict quality control following SOP (Standard Operating Procedures) at a GMP facility.
- High safety: The reprogramming method for iPSC generation employs mRNA, eliminating the risk of genomic insertion, minimizing transgene residuals, and ensuring exceptional safety standards.
Our clinical-grade iPSCs are at the forefront of regenerative medicine, empowering companies and research institutes globally to develop innovative therapies.
A prime example is Gameto's Fertilo technology, which leverages our StemRNA™ Clinical Seed iPSC Clones to create high-purity ovarian support cells for ex vivo egg maturation (Cell Stem Cell, 33, 1-15 (2026)). Fertilo has achieved a significant milestone as the first iPSC-based therapy to receive IND approval and advance to U.S. Phase 3 clinical trials.
Related Products
The items below are all available from the REPROCELL product catalog:
Want to use iPSCs in a clinical context?
GMP iPSC & iMSC MCB Manufacturing Service
REPROCELL’s scientists manufacture GMP iPSC & iMSC Master Cell Banks (MCBs) that are compliant with the regulatory standards and guidelines of the FDA, EMA, and PMDA.
Discover our clinical stem cell services
GMP iPSC/iMSC MCB Manufacturing – REPROCELL USA
GMP iPSC/MSC MCB Manufacturing – Histocell (Europe Partner Overview)
Discover more
Gene Editing Services
- GMP iPSC Production Service
- GMP iMSC & MSC Production Service
- iPSC-Derived Exosome Production Service
- GMP iPSC/iMSC MCB Manufacturing – REPROCELL USA
- GMP iPSC/MSC MCB Manufacturing – Histocell (Europe Partner Overview)
- REPROCELL Japan GMP Manufacturing Facility
- For clinical use: Ready-to-use GMP MSCs
Resources
- FAQ: Clinical iPSCs
- FAQ: Clinical MSCs
- FAQ: iPSC-Derived Exosomes
- Making iPSC-Derived Therapeutics a Clinical Reality – our external article in the European Biopharmaceutical Review.
On the REPROCELL blog
Latest in Stem Cells

REPROCELL Attends Japan-Maryland Night 2026
REPROCELL was honored to attend Japan-Maryland Night on Wednesday March 4th 2026 Maryland State, USA, hosted by the Embassy of Japan (in Washington DC).
06 March 2026

From StemRNA™ Clinical Seed Clone to GMP-Ready Cell Therapy Programs
Discover how REPROCELL’s StemRNA™ Clinical iPSC Seed Clones streamline the path to GMP-ready cell therapy, simplifying regulatory processes and enabling scalable, high-quality manufacturing.
27 February 2026

iPSC-, MSC- and iMSC-Derived Exosome Therapeutics: The Cell-Free Future of Regenerative Medicine
Explore the future of regenerative medicine with iPSC-, MSC-, and iMSC-derived exosome therapeutics, focusing on their advantages, challenges, and clinical potential.
19 November 2025