- Product Catalog
- Featured
- StemRNA™ Research iPSCs
StemRNA™ Research iPSCs
Off-the-shelf cell lines
Human induced Pluripotent Stem Cells
StemRNA human iPSCs (a.k.a. hiPSCs) provide immediate access to the state-of-the-art Stemgent StemRNA 3rd Gen Reprogramming technology. These cells are ideal for validating the StemRNA 3rd Gen Technology with control cells prior to investing in reprogramming to develop patient-derived cell lines.
Our StemRNA human iPSCs are derived from more than ten healthy and neurodegenerative disease donors with a range of demographic and clinical backgrounds. This means they do not contain vectors, viruses, or DNA – unlike iPSCs created using other reprogramming methods.
StemRNA human iPSCs are research-ready and free for commercial applications*, including vaccine testing and drug screening.
(*Terms & conditions apply.)
Browse the full range of StemRNA hiPSCs
Benefits of StemRNA human iPSCs
- Ready for use in experiments such as differentiation
- Reprogrammed using the state of the art StemRNA 3rd Gen Reprogramming Technology
- No specialized reprogramming knowledge required
- No retention or integration of reprogramming vectors
- Imunologically (ICC) and functionally (teratoma formation) pluripotent
- Normal karyotype
- Significant time savings
- Saves 2-4 months or more compared to reprogramming your own iPSCs
- Easy access to iPSCs for start-up labs
- No specialized reprogramming knowledge required
- Grows in standard stem cell media and support matrices
%20vial.jpg?width=185&height=500&name=StemRNA%20human%20iPSCs%20(RCRP004N)%20vial.jpg)
StemRNA™ Human iPSC 802-3G
(cat. no. RCRP004N)
“The StemRNA™ 3rd Gen reprogramming system is outstanding for efficiency and clinical relevance.”
— Dr. Marco Poleganov, Head of Stem Cells & Reprogramming,
BioNTech RNA Pharmaceuticals GmbH
The Science of StemRNA human iPSCs
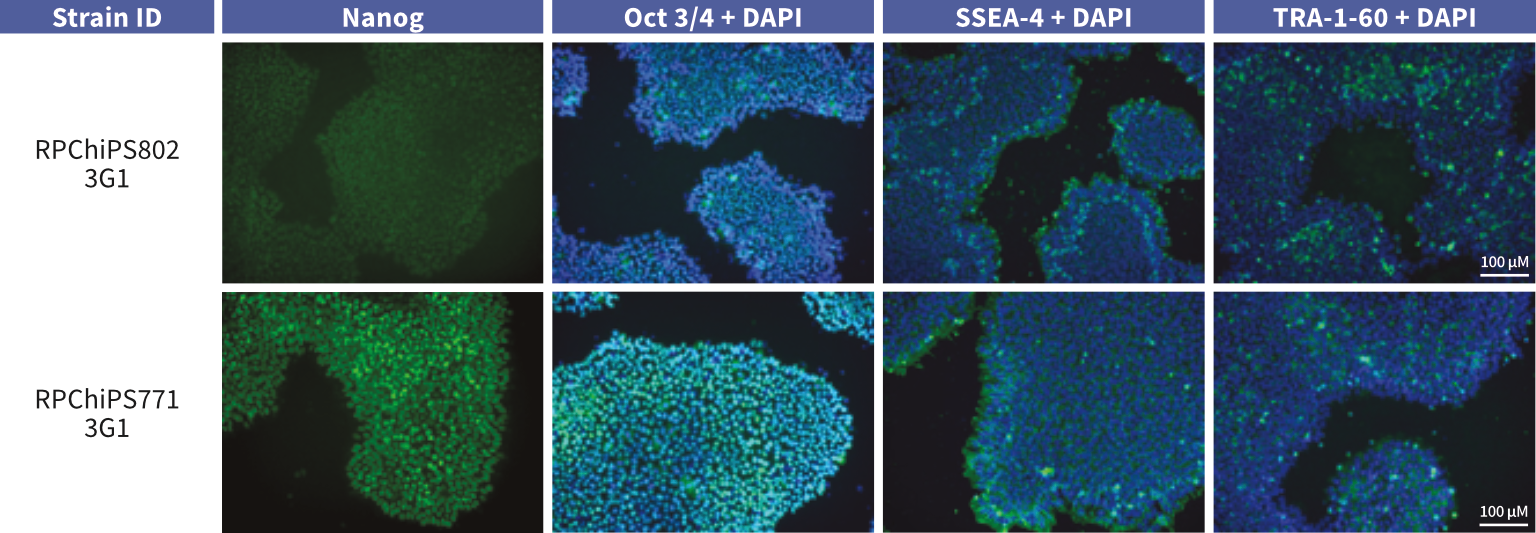
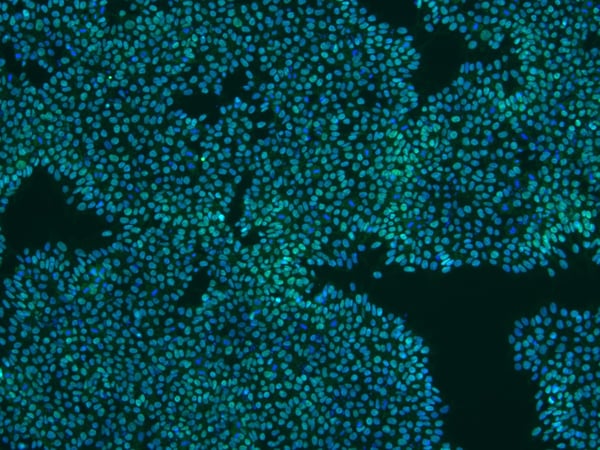
StemRNA human iPSCs grown in vitro stain positive for standard pluripotency markers
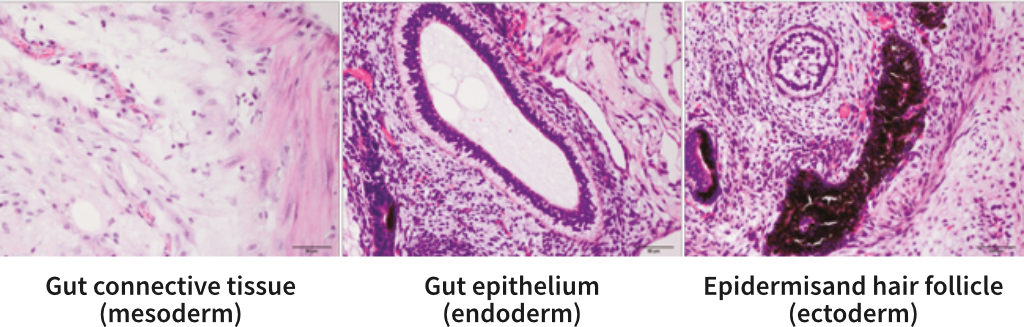
StemRNA human iPSCs differentiate in vivo into all three germ layers
StemRNA human iPSCs exhibit expected karyotype
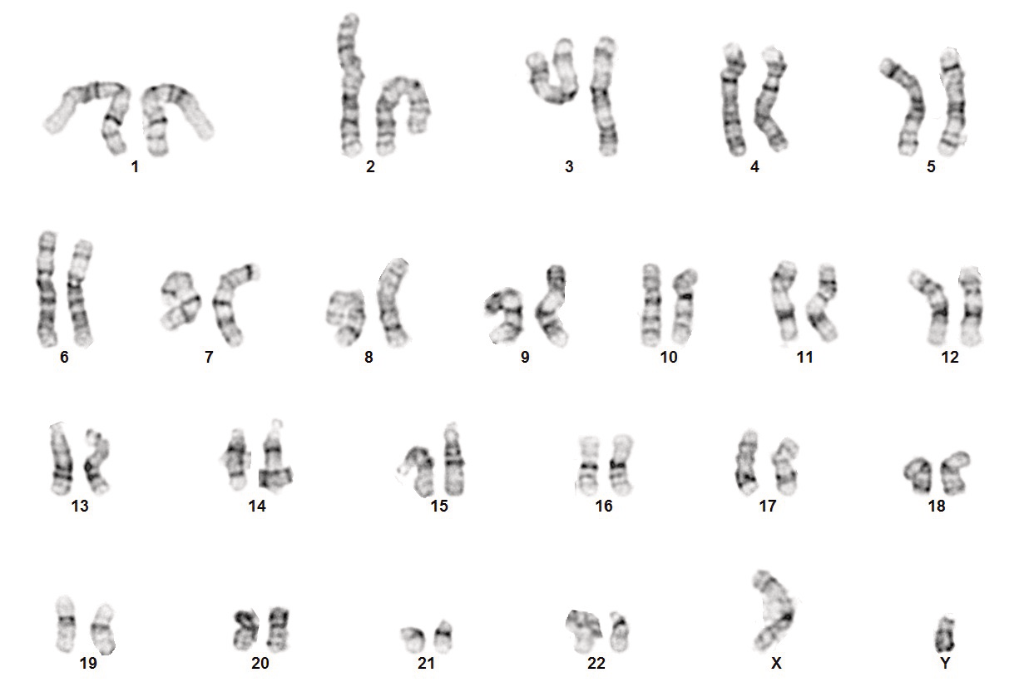
Human iPSCs made using StemRNA 3rd Gen technology
| Cat. No. | Strain ID | Donor Race | Donor Sex | Donor Age | Donor Clinical Status | Reprogramming Technology | Tissue Source |
| RCRP004N | RPChiPS8023G1 | Hispanic | Female | 30 | Healthy | StemRNA 3rd Gen | Blood (EPCs) |
| RCRP005N | RPChiPS7713G1 | Caucasian | Male | 32 | Healthy | StemRNA 3rd Gen | Blood (EPCs) |
| RCRP006N | RPChiPSSK0011 | Asian-Indian | Male | 56 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
| RCRP007N | RPChiPSSK0042 | Asian-Indian | Male | 65 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
| RCRP008N | RPChiPSSK0021 | Asian-Indian | Female | 58 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
| RCRP009N | RPChiPSBL003 | Asian-Indian | Female | 20 | Healthy | StemRNA 3rd Gen | Blood (EPCs) |
| RCRP010N | RPChiPSSK0053 | Caucasian | Male | 56 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
| RCRP011N | RPChiPSSK0032 | Asian-Indian | Female | 20 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
| RCRP012N | RPChiPSSK006 | Filipino | Male | 30 | Healthy | StemRNA 3rd Gen | Skin (Fibroblasts) |
Research Stem Cell Services
If you can't find what you need in the table above, REPROCELL can create custom iPSCs specifically for your project. Service options include:
- Custom collection of starting tissue / target cells to meet your donor criteria
- Reprogramming using our StemRNA 3rd Gen Reprogramming technology
- Expansion, banking, characterization
- Differentiation if required
Related Products
The items below are all available from the REPROCELL product catalog:
Looking for custom clinical iPSCs?
GMP iPSC Master Cell Bank Manufacturing Service
REPROCELL’s scientists manufacture GMP iPSC Master Cell Banks that are compliant with the regulatory standards and guidelines of the FDA, EMA, and PMDA.
Make an inquiry about StemRNA iPSC reprogramming technology
On the REPROCELL blog
Latest in Stem Cells

From StemRNA™ Clinical Seed Clone to GMP-Ready Cell Therapy Programs
Discover how REPROCELL’s StemRNA™ Clinical iPSC Seed Clones streamline the path to GMP-ready cell therapy, simplifying regulatory processes and enabling scalable, high-quality manufacturing.
27 February 2026

iPSC-, MSC- and iMSC-Derived Exosome Therapeutics: The Cell-Free Future of Regenerative Medicine
Explore the future of regenerative medicine with iPSC-, MSC-, and iMSC-derived exosome therapeutics, focusing on their advantages, challenges, and clinical potential.
19 November 2025

Current Landscape of FDA Stem Cell Approvals and Trials 2023-2025
Discover the latest advancements and FDA approvals in stem cell therapies, including iPSC and MSC treatments, reshaping the 2023-2025 clinical landscape.
02 September 2025