Vasodilatation in human subcutaneous resistance arteries (Vasopressin & oxytocin receptors)
Drug Discovery Assay – reference number: B017
Overview
| Assay type: | Blood vessels |
| Tissue: | Human subcutaneous resistance artery (healthy) |
| Target: | Vasopressin & oxytocin receptors |
| Control compound: | Desmopressin |
| Study type: | Wire myography |
| Functional endpoint: | Vasodilatation |
Assay Description
The experiment assesses whether test articles cause vasodilatation in human subcutaneous resistance arteries with Desmopressin as a reference compound.
Resistance arteries are involved in regulating organ specific blood flow and offer the greatest resistance to blood flowing into tissues. Resistance arteries therefore play an important role in the regulation of peripheral vascular resistance and arterial blood pressure.
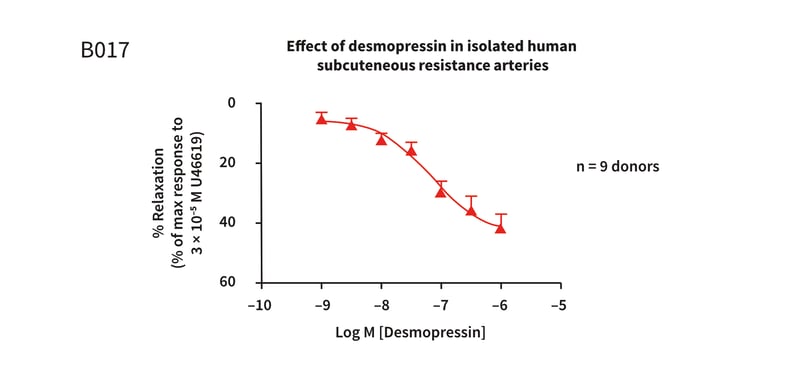 Figure 1: Cumulative concentration response curve to desmopressin in isolated human subcutaneous resistance arteries, pre-constricted with 3 × 10−5M U46619, demonstrating concentration dependent relaxation. The Log EC50 = −7.17 ± 0.20M (67nM) (mean ± S.E.M), with a maximum relaxation response of 42.94% at 1 × 10−6M.
Figure 1: Cumulative concentration response curve to desmopressin in isolated human subcutaneous resistance arteries, pre-constricted with 3 × 10−5M U46619, demonstrating concentration dependent relaxation. The Log EC50 = −7.17 ± 0.20M (67nM) (mean ± S.E.M), with a maximum relaxation response of 42.94% at 1 × 10−6M.
Testing Information
Introduction
The specific results that will be provided are the effects of increasing concentrations of test articles on the contractile state of human isolated subcutaneous resistance arteries.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in distilled water unless otherwise requested. Bath volumes are 5mL; sponsor to provide sufficient test article to run the entire study.
Suggested Testing
In duplicate at 6 concentrations.
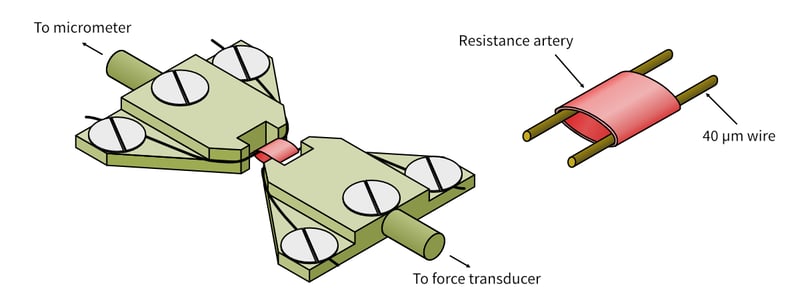
Figure 2: The myograph consists of two stainless steel “jaws”. One jaw is connected to a movable micrometer, the other is connected to a highly sensitive force transducer capable of measuring micro-Newton changes in force. To attach a small artery to the myograph jaws, two thin wires are carefully placed through the lumen of the artery. The artery (with the wires) is then placed between the jaws by attaching one wire to the micrometer jaw and the other to the force transducer jaw. The artery is stretched at a physiologically relevant resting stretch and the stretch stays constant through the experiment (“isometric”). Changes in force detected at the transducer reflect active vasoconstriction or vasodilation of the artery (the force changes, but the stretch stays constant).
Study Outline
Rationale and Experimental Design
The experiment assesses whether test articles cause vasodilatation in human subcutaneous resistance arteries with Desmopressin as a reference compound.
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue. Furthermore, tissues which do not respond to the standard pharmacology checks will be excluded.
Standardisation and Qualification
All individual vessels are initially processed through standardisation and qualification procedures to ensure functionality, prior to starting the study protocol.
Vessels are processed through a standardisation procedure to reduce signal variability prior to pharmacological intervention. This ensures that vessels are maintained under appropriate physiological tension throughout the experiments.
Following standardisation, vessels are “woken up"by 2 successive high potassium salt solution challenges with washes and recovery periods following each challenge. This process standardises the basal resting tone and ensures robust contractile responses.
Vessels are then challenged with an appropriate vasoconstrictive agent before endothelial function is assessed by the addition of the appropriate endothelial dependent vasorelaxant agent. Test tissues are then washed, and the responses allowed to return to baseline.
Vessels which pass the standardisation and qualification pass/fail criteria will then progress to the study protocol
Agonist Relaxation Assays
To assess the ability of each item to elicit vasodilatation, 6 point cumulative concentration response curves (CCRC’s) will be performed for each test item. These CCRC’s will be performed following pre-constriction with an appropriate vasoconstrictive agent. A positive control compound and representative test article vehicle CCRC will also be run to allow direct comparison with test articles.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate vessels):
-
Representative test article vehicle CCRC
-
Test article 1 CCRC
-
Test article 2 CCRC
-
Test article 3 CCRC
-
Positive control CCRC
Supplementary Option
To assess the involvement of a specific receptor subtype in any observed responses, the concentration of test article eliciting the largest response can subsequently (following a wash out and recovery period) be tested in the presence of a specific antagonist. This supplementary option will incur an extra charge.
Antagonist Relaxation Assays
Option 1 − IC50 Determination
To assess the ability of each item to antagonise an agonist mediated vasorelaxation response, 6 point cumulative concentration response curves (CCRC’s) will be performed for each test item. These CCRC’s will be performed following pre-constriction and relaxation with an appropriate vasorelaxant reference agonist. A positive control compound and representative test article vehicle CCRC will also be run to allow direct comparison with test articles.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate vessels):
-
Representative test article vehicle CCRC
-
Test article 1 CCRC
-
Test article 2 CCRC
-
Test article 3 CCRC
-
Positive control CCRC
Option 2 − PA2 determination
To assess the ability of a test item to antagonise an agonist mediated vasorelaxation response; 6 point cumulative concentration response curves (CCRC’s) will firstly be performed for the reference agonist, following pre-constriction with an appropriate vasoconstrictive agent.
Following a wash out and recovery period, vessels will be incubated with the test item (3 different concentrations of test item will be assessed), positive control or test article vehicle before the same 6 point cumulative concentration response curve will be repeated for the reference agonist.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate vessels):
-
Representative test article vehicle
-
Test article 1 concentration 1
-
Test article 1 concentration 2
-
Test article 1 concentration 3
-
Test article 2 concentration 1
-
Test article 2 concentration 2
-
Test article 2 concentration 3
-
Test article 3 concentration 1
-
Test article 3 concentration 2
-
Test article 3 concentration 3
-
Positive control
Analysis
Responses shall be expressed either in milligrams or grams tension, or as a % of the maximal contractile response (e.g. KPSS). Statistical analysis (where appropriate) will be performed using GraphPad Prism, with the results being shown in graphical form in the final report.
.jpg?width=756&height=425&name=Untitled%20design%20(10).jpg)

