Skin Culture Induced Acne Model (LPS)
Drug Discovery Assay – reference number: B111
| Assay type: | Skin |
| Tissue: | Human skin biopsies (Healthy) |
| Target: | TLR4/TLR2 pathway/ glucocorticoid receptors/ NLRP3 inflammasome |
| Control compound: | Betnovate/ nitric oxide |
| Study type: | Ex vivo cultures |
| Functional endpoint: | Cytokine release |
This experiment assesses whether test articles cause a reduction in inflammatory cytokine release in acne, with betamethasone as a reference compound. It uses healthy skin punch biopsies which are induced to display an acne phenotype using lipopolysaccharide (LPS), which activates the TLR2/TLR4 pathway. We then explore test article effect on the expression on inflammatory cytokines via ELISA and/or rtPCR.
[POSTER] Case study by Novan using this induced-acne model →
Testing Information
The specific results that will be provided are on the anti-inflammatory effect of test articles across an induced acne model using human fresh skin.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in deionized water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
We suggest test conditions are assessed in triplet as a minimum.
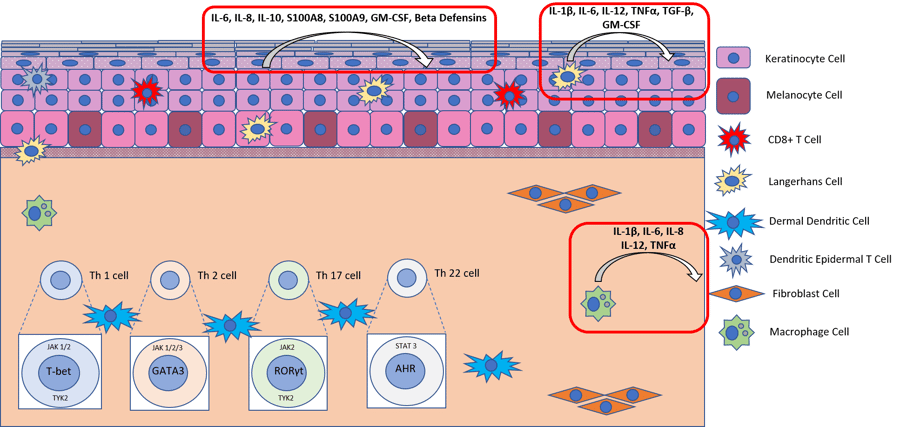
Diagram of the molecular pathway activated by LPS in healthy skin.
Study Outline: Rationale and Experimental Design
Option 1: Systemic administration of test article
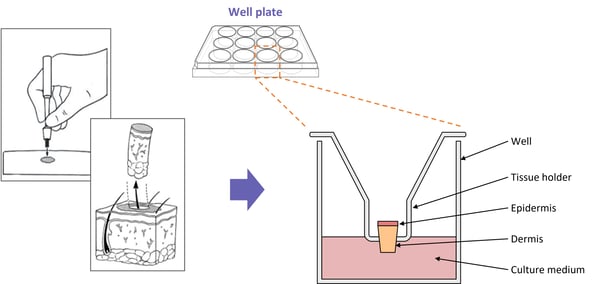
Full-thickness skin biopsies are achieved via punch-biopsy of residual surgical tissue. Biopsies are taken at a size of 3mm2 and transferred onto a mesh transwell in a 12 well plate. The dermis of the skin is submerged in specially fortified media, leaving the epidermis exposed to air. Biopsies are incubated in optimum conditions for a maximum of 24 hrs, then cultured in the presence of the inflammatory cocktail and the test article for a further 24 hrs. Tissue media is collected for phenotype analysis by ELISA and/or the biopsy set is collected for rtPCR.
An example of the conditions assessed for 3 test articles are detailed below (it is recommended a minimum of triplicates should be used for each condition):
- Non-stimulated control
- Stimulated control
- Test article 1
- Test article 2
- Test article 3
- Positive control
Option 2: Topical application of test article
Full-thickness skin biopsies are achieved via punch-biopsy of residual surgical tissue. Biopsies are taken at a size of 8mm2 and transferred onto a mesh transwell in a 12 well plate. The dermis of the skin is submerged in specially fortified media, leaving the epidermis exposed to air. Biopsies are incubated in optimum conditions for a maximum of 24 hrs, then cultured in the presence of the inflammatory cocktail (added to the media) and the test article (added to the exposed skin surface) for a further 24 hrs. Tissue media is collected for phenotype analysis by ELISA and/or the biopsy set is collected for rtPCR.
An example of the conditions assessed for 3 test articles are detailed below (it is recommended a minimum of triplicates should be used for each condition):
- Non-stimulated control
- Stimulated control
- Test article 1
- Test article 2
- Test article 3
- Positive control
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue.
Standardization and Qualification
Tissue that does not respond to stimulation with LPS would be deemed non-functional.
Analysis
Cytokine analysis: Supernatant samples can be analyzed for specific analytes of your choice by a multiplex ELISA platform. Each analyte will be quantified by interpolation against a standard curve generated on the same 96 well analysis plate. Other forms of end point analysis are available such as gene expression or immunohistochemistry.
Example data from our induced acne model
Induction of inflammatory cytokine secretion after 24 hrs
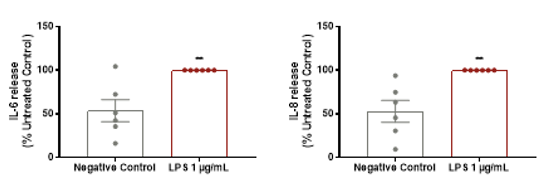 Lipopolysaccharide (LPS) was found to significantly increase cytokine release in our induced acne model compared with normal control. Data is presented as mean ± SEM in bar format with the individual donor means presented in the scatter. Analysis was conducted by unpaired two-tailed t-test. Six subjects were evaluated in triplicate measurements. Asterisks indicate significant (P < 0.05, for one, P < 0.01 for two, P < 0.001 for three and P < 0.0001 for four) differences versus the negative control group.
Lipopolysaccharide (LPS) was found to significantly increase cytokine release in our induced acne model compared with normal control. Data is presented as mean ± SEM in bar format with the individual donor means presented in the scatter. Analysis was conducted by unpaired two-tailed t-test. Six subjects were evaluated in triplicate measurements. Asterisks indicate significant (P < 0.05, for one, P < 0.01 for two, P < 0.001 for three and P < 0.0001 for four) differences versus the negative control group.
Inhibition of inflammatory secretion by corticosteroid treatment
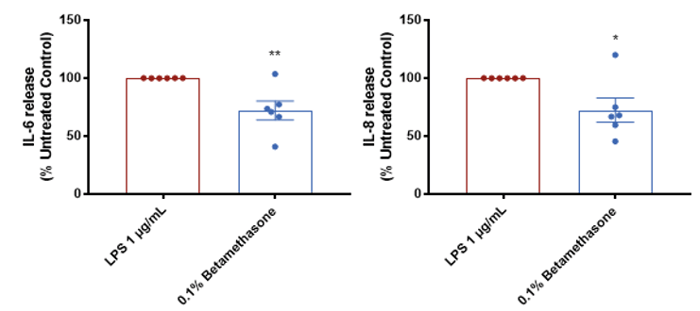 Steroid treatment was found to significantly reduce cytokine release in our acne model compared with the untreated control. Data is presented as mean ± SEM in bar format with the individual donor means presented in the scatter. Analysis was conducted by unpaired two-tailed t-test. Six subjects were evaluated in triplicate measurements. Asterisks indicate significant (P < 0.05, for one, P < 0.01 for two) differences versus the negative control group.
Steroid treatment was found to significantly reduce cytokine release in our acne model compared with the untreated control. Data is presented as mean ± SEM in bar format with the individual donor means presented in the scatter. Analysis was conducted by unpaired two-tailed t-test. Six subjects were evaluated in triplicate measurements. Asterisks indicate significant (P < 0.05, for one, P < 0.01 for two) differences versus the negative control group.


