Example Protocol for the Culture of the Breast Invasive Ductal Carcinoma BT-474 Cell Line on Alvetex® Scaffold in Well Insert and Well Plate Formats
● Download this protocol as a PDF (2 MB)
1. Introduction
BT-474 is a human invasive ductal carcinoma cell line isolated in the late 1970s from breast cancerous tissue[1]. BT-474 cells are characterised by the overexpression of epidermal growth factors receptors[2], as well as the presence of progesterone receptors and mutant p53[3]. This expression profile makes them a good model for HER2+ or progesterone-responsive tumours and they have been used to these ends in both cytotoxicity studies[4] and as xenografts in rodents[5,6].
Alvetex Scaffold is available in several cell culture formats including 24 well plate (AVP006), 12 well plate (AVP002), 6 well insert (AVP004), 12 well insert (AVP005), and 24 well insert (AVP012).
24 well and 12 well plates are suitable for shorter term cultures and for applications where limited cell penetration into the scaffold is required. Well insert formats generally support longer term cultures and deeper cell penetration into the scaffold. They also provide for conveniently tailored media set ups (see the Alvetex Scaffold Quick Start protocol).
The availability of two different well insert formats enables choice on the basis of desired culture size and cell expenditure. 6 well inserts can be placed in conventional 6 well plates, while 12 well inserts can be placed in either 6 well plates or 12 well plates, depending on media requirements. Alternatively, both insert types can be housed in the dedicated Well Insert Holder in Deep Well Petri Dish (AVP015) to allow for increased media volumes and prolonged cell culture.
2. Methods
2.1. Preparation for 3D Cell Culture on Alvetex Scaffold
- BT-474 cells (ATCC, HTB-20) were routinely maintained in T-75 flasks.
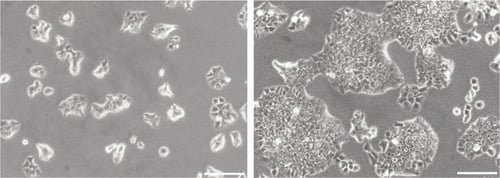
Figure 1. Phase contrast micrographs of 3T3 cells grown in conventional 2D culture plates. Images show cells at low (left) and high (right) confluency. Scale bars: 200 µm.
-
Complete growth media consisted of: RPMI1 640 supplemented with 10 % v/v FBS, 2 μM L- glutamine and 100 U/mL Penicillin/Streptomycin.
-
Cells were harvested by trypsinisation and centrifuged for 5 minutes (1000 rpm). The supernatant was discarded and the cell pellet was re-suspended in an appropriate volume of media for cell counting by Trypan Blue.
-
Cells were re-suspended at a concentration of 1.0 × 106 cells/mL for seeding.
2.2. 24 well Plate Format (AVP006)
-
Alvetex Scaffold 24 well plates were prepared for seeding with a 70 % ethanol wash (2 mL per well) and subsequent media washes (twice with 2 mL of media each).
-
0.5 mL of the cell suspension was added to the centre of the Alvetex Scaffold disc, which was equivalent to 0.5 × 106 cells per well.
-
The plate was incubated for at least 1 hour at 37 °C with 5 % CO2 to allow the cells to settle into the scaffold.
-
1.5 mL of media was added to each well taking care not to dislodge cells from the Alvetex Scaffold.
-
Plates were re-incubated and maintained by complete media exchange every other day.
Note: This method can be applied to the use of Alvetex Scaffold in 12 well plate format (AVP002). Adjust cell seeding and media volumes according to the guidelines provided in the Alvetex Scaffold Quick Start protocol.
2.3. 6 well Insert Format (AVP004)
-
Alvetex Scaffold 6 well inserts in 6 well plate format were prepared for seeding by dipping in 70 % ethanol followed by media washes (twice with 10 mL per well).
-
Wells were filled from the outside of the insert with enough medium to allow it to rise inside the insert and cover the substrate, but not to go over the sides of the inserts, i.e. 7 mL ± 1 mL, as described for feeding above and below separately in the Quick Start Protocol.
-
1 mL of the cell suspension was added in droplets over the surface of the Alvetex Scaffold disc, which was equivalent to 1.0 × 106 cells per well.
-
The plate was incubated overnight at 37 °C with 5 % CO2 to allow the cells to settle into the scaffold.
-
Media was added to each well to a total volume of 10 mL the following morning taking care not to dislodge cells from the Alvetex Scaffold.
-
Plates were re-incubated and maintained by complete media exchange every other day.
Note: This method can be applied to the use of Alvetex Scaffold in 12 well insert format (AVP005). Adjust cell seeding and media volumes according to the guidelines provided in the Alvetex Scaffold Quick Start Protocol.
3. Example Data
3.1. 24 well plate format (AVP006)
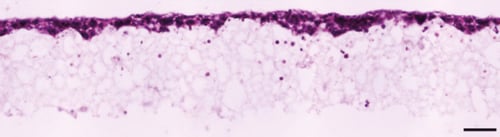
Figure 2. Brightfield micrograph showing the structure of BT-474 cells cultured for 7 days on 15 mm diameter Alvetex Scaffold discs presented in 24 well plate format. Cells were fixed, embedded in paraffin wax, sectioned (10 µm) and counterstained with haematoxylin and eosin. Scale bar: 100 µm.
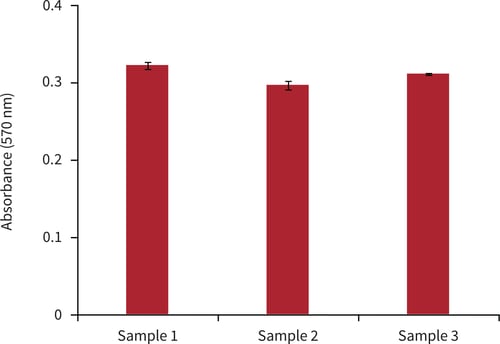
Figure 3. Biochemical analysis of cell viability using a standard MTT assay. Data from 3 sample replicates of BT-474 cells are shown, each sampled in triplicate (n = 3, mean ± SD). Cells were cultured for 3 days on 15 mm Alvetex Scaffold discs presented in 24 well plate format.
3.2. 6 well insert format (AVP004)
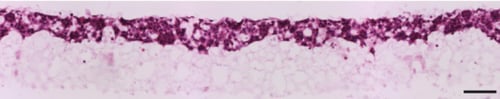
Figure 4. Brightfield micrograph showing the structure of BT-474 cells cultured for 7 days on 22 mm diameter Alvetex Scaffold discs presented in 6 well insert in 6-well plate format. Cells were fixed, embedded in paraffin wax, sectioned (10 µm) and counterstained with haematoxylin and eosin. Scale bar: 100 µm.
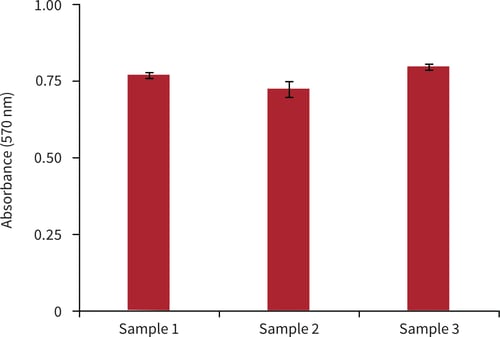
Figure 5. Biochemical analysis of cell viability using a standard MTT assay. Data from 3 sample replicates of BT-474 cells are shown, each sampled in triplicates (n = 3, mean ± SD). Cells were cultured for 3 days on 22 mm Alvetex Scaffold discs presented in 6 well inserts in 6-well plate format.
4. References
-
Lasfargues EY et al, 1978. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst 61(4):967-78.
-
Imai Y et al, 1982. Epidermal growth factor receptors and effect of epidermal growth factor on growth of human breast cancer cells in long-term tissue culture. Cancer Res 42(11):4394-8.
-
Liang Y et al, 2005. p53-dependent inhibition of progestin-induced VEGF expression in human breast cancer cells. J Steroid Biochem Mol Biol 93(2-5):173-82.
-
Baselga J et al, 1998. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58(13):2825-31.
-
Liang Y et al, 2007. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res 67(20):9929-36.
-
Nanni P et al, 2012. Multiorgan metastasis of human HER-2+ breast cancer in Rag2–/–; I l2rg– /– mice and treatment with PI3K inhibitor. PLoS One 7(6): e39626.