The Science of Alvetex
Introduction
The Alvetex membrane and its associated bespoke plasticware is an innovative and novel solution that enables researchers and product developers to undertake new ways of culturing cells and tissues in vitro.
Alvetex is a market leading scaffold product. Not only does Alvetex enable simple and routine 3D cell culture, but it also empowers users to bioengineer tissue constructs, creating unique models of human tissues. This opportunity can improve the predictive accuracy of in vitro models, that in turn decreases costs and enhances efficiency of bringing therapeutic and cosmetic products to market.
Here we tell the story behind the science of Alvetex technology and highlight its compatibility and applications.

Above: A: Alvetex 6 well insert containing Alvetex Scaffold (AVP004). B: Alvetex Advanced 15 mm modular well insert containing Alvetex Scaffold (AVP022). C: Alvetex Scaffold disc, 200 μm thickness. D: Scanning Electron micrograph of Alvetex Scaffold, transverse section.
Alvetex 3D cell culture technology was originally pioneered in the laboratory of Professor Stefan Przyborski at Durham University, UK, in the early 2000’s. Prof. Przyborski founded the company Reinnervate Ltd in 2002 with the purpose of commercializing and further developing Alvetex products and services. REPROCELL Inc. (Japan) acquired Reinnervate in 2014.
The development and production of Alvetex and a two ranges of plasticware (the Alvetex 3D Cell Culture Systems and the new (2025) Alvetex Advanced Tissue Bioengineering System) continues at REPROCELL Europe Ltd, together with Alvetex-based Bioengineered 3D Tissue Models that are now part of the services offered by REPROCELL's Preclinical and Drug Discovery Contract Research Organization (CRO).
Prof. Stefan Przyborski is now a Chief Scientific Officer (CSO) of REPROCELL Europe as well as being a Professor in the Department of Biosciences at Durham University, UK. Together with his team, Prof Przyborski continues to improve and develop Alvetex, its supporting bespoke plasticware products, and 3D cell culture and bioengineered tissue services.
▶ Read more about the history of Alvetex.
▶ Read some testimonials from scientists who recommend Alvetex.
The Alvetex Membrane
The Alvetex membrane is currently available in two types: Alvetex Scaffold and Alvetex Strata. Both materials are presented as 200 µm thick membranes of highly porous cross-linked polystyrene. The difference is in their fine structure and architecture.
Alvetex Scaffold
Alvetex Scaffold is primarily designed for three dimensional culture of dissociated mammalian cells within the scaffold, forming three dimensional associations as they propagate and migrate.


Above: Scanning electron microscope image of Alvetex Scaffold, to highlight its porous structure. A: a 200 µm thick Alvetex Scaffold disc. B: Close up of Alvetex Scaffold voids with dimensions of approximately 42 µm in diameter and interconnects of approximately 13 µm in diameter.
Alvetex Strata
Alvetex Strata is primarily designed to support the growth of cells and intact tissues on the surface of the membrane.


Above: Scanning electron microscope image of Alvetex Strata. C: a 200 µm thick Alvetex Strata disc. D: Close up of Alvetex Strata voids with dimensions of approximately 15 µm in diameter and interconnects of approximately 5 µm in diameter.
Features and Benefits of Alvetex
| Alvetex Feature | Alvetex Benefits |
|---|---|
| Same polystyrene as existing cell culture plasticware |
|
| Consistent scaffold structure – extremely low batch to batch variability |
|
| Entire scaffold is only 200 µm thick |
|
| > 90 % Porosity |
|
| Alvetex Scaffold void dimensions approximately 42 µm |
|
| Alvetex Strata void dimensions approximately 15 µm |
|
Alvetex resources online
In the Resources section of the REPROCELL website we have a wide range of materials for scientists who are interested in using Alvetex:
Many scientists around the world use Alvetex with success in their research. This research has generated significant amounts of data which has been published extensively in peer-reviewed journals. This body of scientific literature continues to grow year on year, and it exemplifies the use of Alvetex in multiple applications with many cell and tissue types.
Alvetex: Genuine 3D Cell culture
Conventional 2D vs Alvetex 3D Cell Culture
In order to enable survival in 2D culture, cells are forced to make dramatic changes to their morphology. Gene expression mediated changes to the cytoskeleton result in a flattened cell morphology. These changes can impair cellular functions.
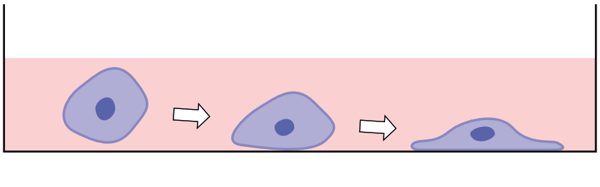
| Cell Detects ECM, membrane proteins and cytoskeleton react to changes in the environment  |
Cell Responds Triggers changes in gene expression and subsequent remodeling of cytoskeleton  |
Cell Adapts Cell shape changed; cell interactions are lost; change in cell function
|
Consequently, cells cultured in the laboratory in 2D usually do not grow and function in a realistic fashion. This has major implications for research and discovery. For example:
- Inaccuracy of predictive assays in the drug discovery process
- Modification of normal behavior of cells in response to external stimuli
- Generation of potentially inaccurate / misleading data
- Misunderstanding of complex biological phenomena
- Poor planning and direction of future research program
Add Back the Third Dimension in Vitro With Alvetex
The structure of Alvetex provides cultured cells with an environment and physical space in which to grow in three dimensions. The architecture of Alvetex, as viewed by a scanning electron microscope, illustrates voids that are interconnected by pores creating a material with > 90% porosity. In the production of Alvetex, emulsion templating is used to control the size of the voids, optimizing the porosity of the material for 3D cell culture. The matrix structure has been designed to enable cells to reproduce an environment that is more consistent with the in vivo cellular environment.
| Conventional 2D Cell Culture | Alvetex 3D Cell Culture |
A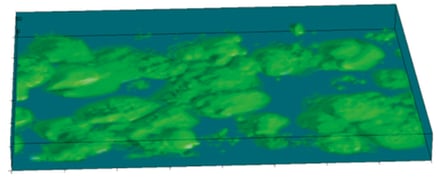 |
B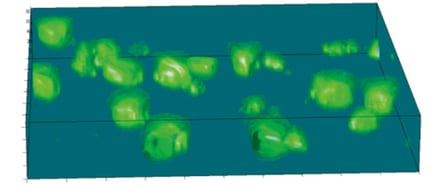 |
| C |
D |
Above: Cells grown on conventional 2D surfaces (A and B) adopt a typical flattened morphology covering a large surface area in horizontal x–y plane (A) and have a reduced height in the vertical z plane (B). In comparison, cells maintained in Alvetex Scaffold (C and B) retain a more cuboidal morphology and 3D cell structure, particularly in the z-plane.
Data generated during a collaborative project between Reinnervate Ltd and LGC Standards – data now published in the following journal: Title: Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three dimensional culture on a novel polystyrene scaffold designed for routine use. Maaike Schutte, Bridget Fox, Marc Baradez, Alison Devonshire, Jesus Minguez, Maria Bokhari, Stefan Przyborski, Damian Marshall. Assay and Drug Development Technologies. DOI: 10.1089/adt.2011.0371. (Reinnervate Ltd. was acquired by REPROCELL Inc. in 2014, and merged with Biopta Ltd. to form REPROCELL Europe Ltd. in 2016.)
A. A cell grown in traditional 2D culture plasticware.

B. A cell grown in Alvetex Scaffold.

Above: Electron microscope images showing how a cell deforms to adapt to a 2D tissue culture environment (A), versus how a cell appears in Alvetex 3D cell culture (B).
A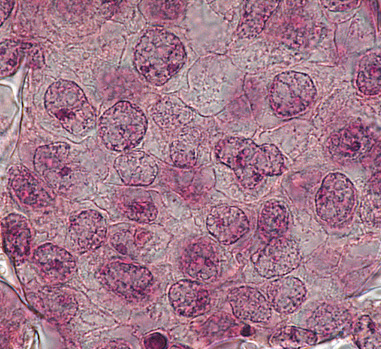
B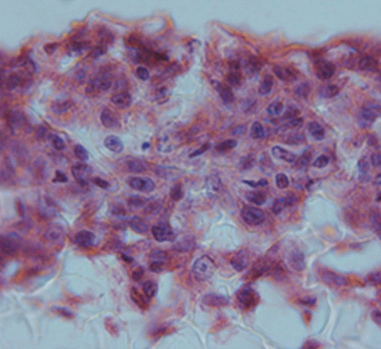
Above: Cells grown within Alvetex Scaffold maintain their natural shape and 3D organization. A: 3D cell culture of human pluripotent stem cells within Alvetex Scaffold. B: 3D cell culture of liver hepatocytes grown within Alvetex Scaffold.
Cell Morphology
Unlike cells grown on conventional 2D substrates where cell morphology is much more varied in appearance, consisting of clumps and individual flattened cells, cells grown in Alvetex exhibit a morphology that is consistent with that found within the in vivo environment. The appearance of cells is more homogeneous with a high degree of 3D organisation.
Typical mammalian cells are around 10-25 μm in size and are rarely further than 0-50 μm from another cell or 100-200 μm from a source of nutrients via a blood capillary. Alvetex is made of the same polystyrene as used in traditional 2D cell culture plasticware. Alvetex has been designed to enable cells to reproduce natural shape and form to enable the cell biologist to maintain the integrity of the micro-scale in vivo cell environment within simple in vitro models.
| Traditional 2D Cell Culture | Alvetex 3D Cell Culture | Normal in vivo Environment | |
|---|---|---|---|
| General dimensions and physical differences | |||
| Maximum distance of cell from the source of nutrients | 0 | 0-100 μm | 0-200 μm |
| Resemblance to in vivo cellular environment | Low | High | N/A |
| Appearance of cell morphology | Flattened | 3D shape | 3D shape |
| Potential for 3 dimensional cell to cell interactions | Very low | High | High |
| Ability to form complex 3 dimensional cellular structures | Very low | High | High |
| Upon initial seeding of cells onto plasticware | |||
| Degree of cellular stress placed upon cell structure | High | Low | N/A |
| Changes to protein and gene expression | High | Low | N/A |
| Cell surface area in contact with plasticware | At least 50% | 0-50% | N/A |
| Post seeding phase | |||
| Ongoing changes to cellular shape | High | Low | N/A |
| Need for remodeling of cytoskeletal architecture | High | Low | N/A |
| Deviation from normal in vivo morphology | High | Low | N/A |
| Cell surface area in contact with plasticware | At least 50% | 0-50% | N/A |
| Opportunity for enhanced in vitro cell functionality | Low | High | N/A |
Cell-to-cell Interactions and Organization
Cells grow and divide occupying the 3D space within the porous Alvetex scaffold. Cells form complex interactions with one another, behaving in a manner that far more closely mimics normal growth in tissues than is possible using traditional 2D techniques.
Cells are free to migrate throughout the scaffold, functioning as they would within their natural environment, laying down extracellular matrix and forming organized and complex in vivo-like structures. This often leads to the formation of “mini slabs” of tissue.
Alvetex derived cell cultures can be processed just like normal tissue samples and prepared for histology using standard procedures including fixation, embedding, thin sectioning and counter-staining.
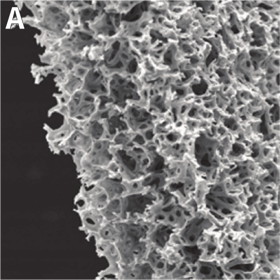
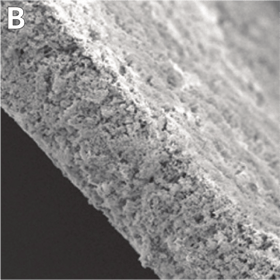
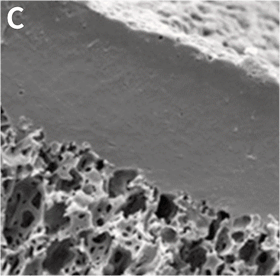
Above: 3D growth of cultured cells form 200 μm thick ‘slabs of tissue’. A: Naked Alvetex Scaffold before cell seeding viewed under scanning electron microscope to show the highly porous scaffold. B: Cells have grown throughout Alvetex Scaffold to the point where the scaffold is no longer visible. C: Hepatocytes form a thick multilayer on top of Alvetex Strata with maximal cell-cell contact as would be found within in in vivo tissue.
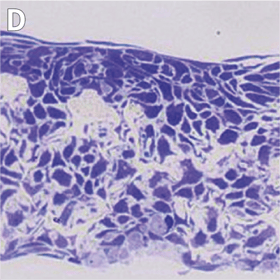
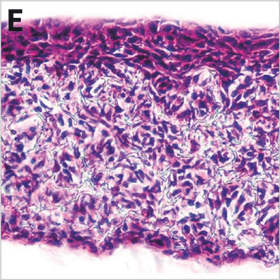
Above: Tissue processing and staining highlight the complex organization of cells growing through-out Alvetex Scaffold. D: Resin sections of Alvetex Scaffold showing skin keratinocytes stained with Toluidine Blue. E: Paraffin sectioned skin keratinocytes counter stained with H&E viewed by bright field microscopy.
Improved Cell Function
Alvetex 3D cell culture enables cells to maintain their natural morphology and and form complex organization, leading to improved cell function and responsiveness which is much more representative of the natural in vivo environment.
Alvetex delivers data of unmatched biological relevance, and provides a much deeper understanding of how cells function in vivo. Factors such as cell viability and responsiveness have been demonstrated to be enhanced when growing cells in Alvetex in comparison to 2D monolayer cultures.
Cell function example 1: Improved Cell Function and Responsiveness
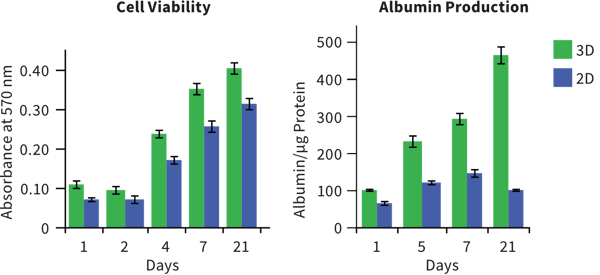
Above: Assessment of HepG2 cells grown on 2D and 3D substrates. Cell viability was determined using a MTT assay and showed greater numbers of viable HepG2 cells in Alvetex Scaffold than on 2D substrates. Similarly, the secretion of albumin from 3D HepG2 cells was elevated compared to standard 2D cultures. In both cases, these data have been normalized to total protein per well to take into account differences in cell numbers. Overall, these results indicate the superior performance of HepG2 cells in 3D culture compared with their 2D counterparts.
Cell function example 2: Increased Cell Viability
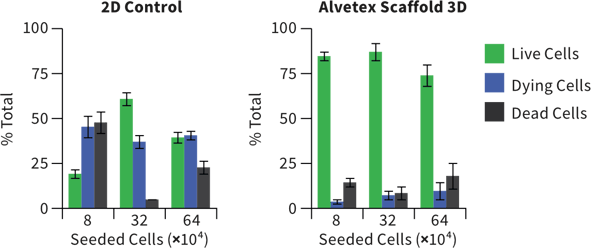
Above: Cell Viability Albumin Production Cell viability of rat primary hepatocytes determined by quantification of live/dead cell staining of hepatocytes maintained for 24 hours on 2D plasticware or Alvetex Scaffold. Cells showed greater than 74% viability when grown on Alvetex Scaffold compared to 2D monolayer culture.*
Cell function example 3: Increased Metabolic Responses

Above: Metabolic responses to model toxicants were significantly enhanced using Alvetex Scaffold. Primary rat hepatocytes were cultured for 3 days in either 2D or 3D culture, Cytochrome p450 expression was induced in cells using a cocktail of model toxicants.*
Example 4: Increased Cell Sensitivity

Above: Primary hepatocytes cultured on 2D plasticware versus Alvetex Scaffold were exposed to a range of acetaminophen (APAP) concentrations for a period of 20 hours and their viability was determined by a standard MTT assay. In general, these data demonstrate that rat primary hepatocytes cultured in 3D using Alvetex Scaffold show increased sensitivity to the model toxicant, acetaminophen.*
* Data generated during a collaborative project between Reinnervate Ltd and LGC Standards – data now published in the following journal: Title: Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three dimensional culture on a novel polystyrene scaffold designed for routine use. Maaike Schutte, Bridget Fox, Marc Baradez, Alison Devonshire, Jesus Minguez, Maria Bokhari, Stefan Przyborski, Damian Marshall. Assay and Drug Development Technologies. DOI: 10.1089/adt.2011.0371
Examples of Cell Types Cultured on Alvetex
Following is an inexhaustive list of examples of cell types that have been successfully grown in Alvetex 3D cell culture. Many of these have been grown by REPROCELL scientists (see Alvetex protocols), while others have been reported in scientific journals (see Alvetex publications).
- 3T3 cells
- HaCaT cells
- HepG2 cells
- TERA2.cl.SP12 cells
- CHO-K1 cells
- LN229 cells
- Full thickness skin equivalent
- SW480 cells
- SW620 cells
- PC3 cells
- BT474 cells
- MG63 cells
- Primary rat MSCs
- MCF-7 cells
- Caco-2 cells, and the co-culture of Caco-2 with CCD-18co cells
- CCD-18co cells
- H1299 cells
- U118-MG glioblastoma cells
- Bone marrow stromal cells
- Primary hepatocytes
- Upcyte® hepatocytes
- Neurospheres
- Adipose tissue-derived stem cells
- Human pluripotent stem cell-derived neurons
- Cylindroma primary cells
- MET4 squamous carcinoma cells
- L929 mouse fibroblasts
- Neural crest cells
- Human nucleus pulposus cells
- Chondrocytes
- Primary chick embryonic tissue
- Brain tissue slices
- Equine oviduct cells
- Spermatogonial stem cells
- Human disc cells
- Melanocytes
- A375/A2058 melanoma cells
- 3T3-L1 cells
- Prostate cancer cells and prostate stem cells
- H9 human embryonic stem cells
- CRL-11372 osteoblasts
- HEK293 human embryonic kidney cells
- MB49 mouse bladder cancer cells
- Rat primary urothelial cells
- Primary retinal pigment epithelium
- Urothelial cells
- Myofibroblasts
- Astrocytes
- Primary and secondary ESC-derived hepatic cells
- Primary keratinocytes
- Pancreatic duct and stromal cells
- GSC glioblastoma stem cells
- b.END-3 endothelial cells
- HCT116 cells
- JJ012 chondrosarcoma cells
- NG108 stem cells
- MBT-2 cells
Alvetex compatibility with biology techniques
Alvetex is compatible with a wide range of cell biology techniques. Following are some examples.
Imaging
Imaging reveals the integrity of in vivo-like structure and organization.
Changes in cellular morphology and function limit the value of cells grown in 2D cell culture. 3D culture systems enable cells to form more complex structures. Various models have been developed to create 3D skin constructs in vitro, including raft cultures. These methods are often technically challenging, involve multiple steps, show poor reproducibility and are difficult to practice routinely.
Alvetex provides an alternative method for 3D growth of keratinocytes, enabling reproduction of natural in vivo structures including the development of the stratum corneum, an essential component of the epidermal barrier. Skin constructs generated on Alvetex can then be used for drug and allergen penetration studies as well as assessment of barrier function.
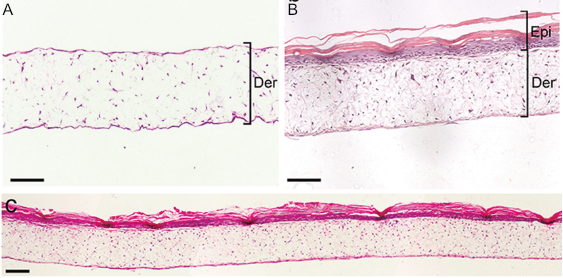
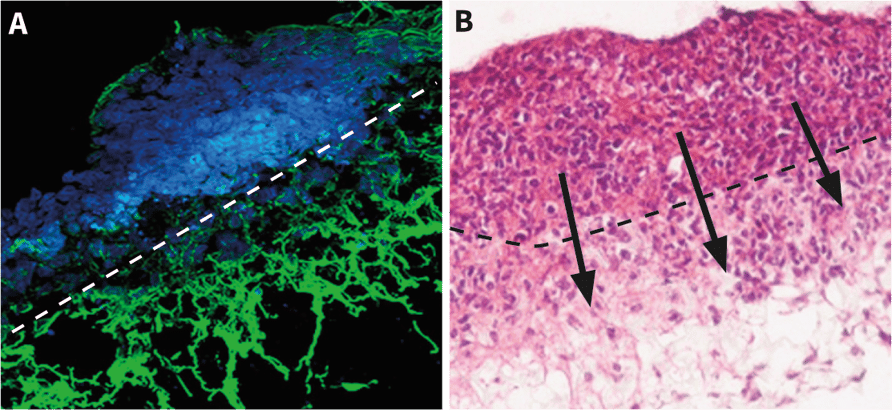
Above: Histology images showing a full thickness skin construct grown by co-culturing human dermal fibroblasts inside Alvetex Scaffold and human keratinocytes on top, thus replicating both the dermal and epidermal compartments. Note that the presence of a stratum corneum is also evident. Validation of dermal and epidermal structure in full-thickness human skin equivalents. A: Representative photomicrographs of haematoxylin and eosin (H&E) stained Alvetex Scaffold seeded with human dermal fibroblasts after culture in Media A for 18 days. B & C: Representative photomicrographs showing H&E stained 35 day full-thickness human skin equivalents at 20× and 10× magnification respectively.*
* Data generated during a collaborative project between Reinnervate Ltd and Newcastle University – data now published in the following paper: A Novel Fully Humanized 3D Skin Equivalent to Model Early Melanoma Invasion. Authors: D.S. Hill, N.D. Robinson, M. P. Caley, M. Chen, E.A. OʼToole, J.L. Armstrong, S. Przyborsky and P.E. Lovat. Mol Cancer Ther. 2015 November ; 14(11): 2665–2673. doi:10.1158/1535-7163.MCT-15-0394.
Resources on Alvetex and imaging techniques
Protocols:
- Cell visualization
- Advanced microscopy:
See also Histology protocols in Sectioning and Counterstaining below.
Whitepapers:
Coating
Alvetex can be coated with extracellular matrix (ECM) proteins and other reagents commonly used to treat cell culture substrates, notably: Collagen I; Collagen IV; Fibronectin; Laminin; Poly-D-lysine; Poly-L-lysine; Poly-D-lysine and Laminin; Poly-L-orthinine and Laminin; Matrigel™; PuraMatrix™.
A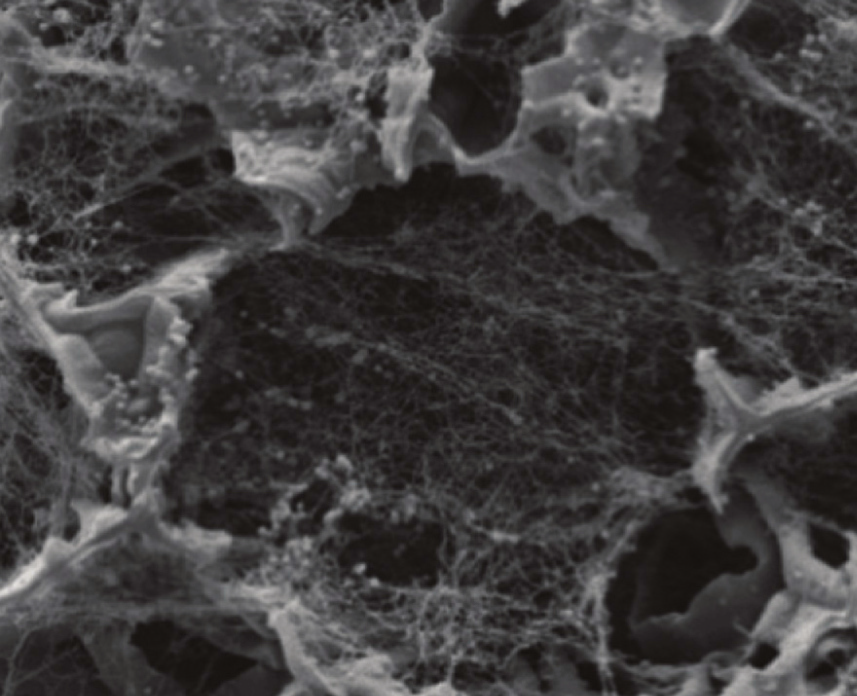
B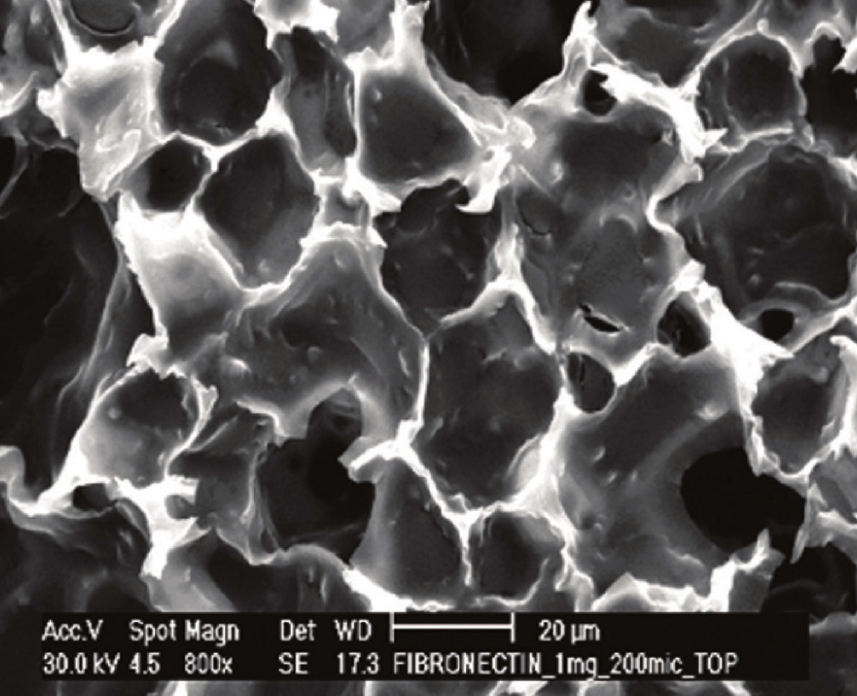
C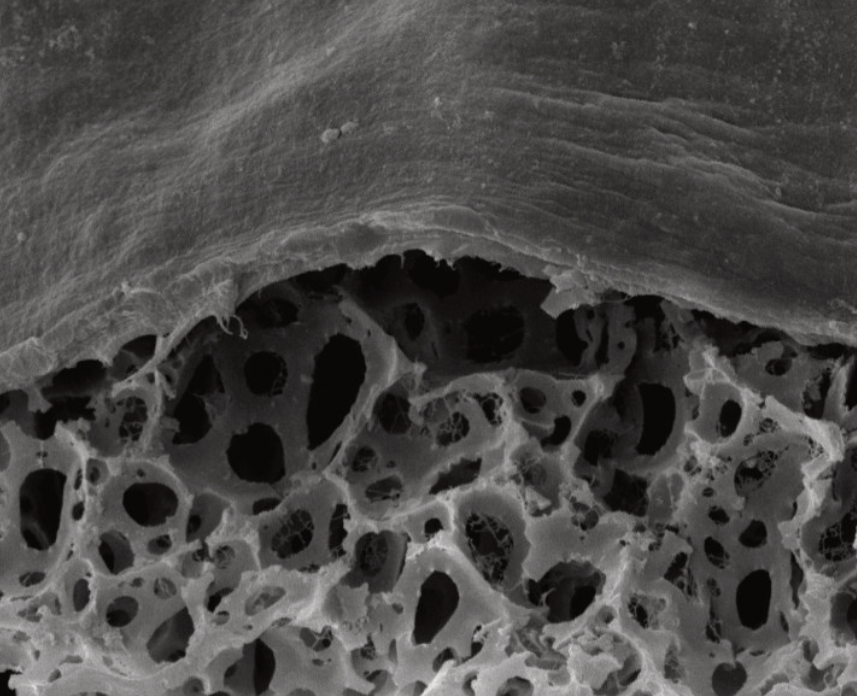
A: Scaffold pre-loaded with Collagen IV. B: Coating Alvetex Scaffold with fibronectin. C: Coating Alvetex Strata with Collagen I (2 mg/mL shown). The ECM proteins form a web of fibers spanning voids into which cells can grow and migrate in 3D. Depending on the ECM concentration used, this coating can either encourage cell invasion into the scaffold or create a barrier between two co-cultured cell populations.
Resources on coating Alvetex for cell culture
Protocols:
Explanting
Directly explanted cells can move into Alvetex directly from pieces of primary tissue or cell aggregates, migrating freely into the scaffold and spreading throughout its structure. Depending on cell type and characteristics, cells may proliferate as well as migrate. By enabling cells to be explanted in this way, Alvetex creates the opportunity for many different applications including tumor cell biology, separation of alternative cell types and establishing and maintaining 3D cultures de novo directly from primary sources, etc.
Above: Examples of cells from tissue pieces placed on top of Alvetex Scaffold migrating into the structure of the scaffold. A: A neural aggregate generated on a low-adherence plate before transfer to Alvetex Scaffold shows extensive neurite elongation within the thickness of the scaffold. B: Cells from an embryonal carcinoma aggregate readily invade Alvetex Scaffold.
Freshly-obtained intact tissues can also be maintained directly on Alvetex Strata for improved adherence and stability during imaging.
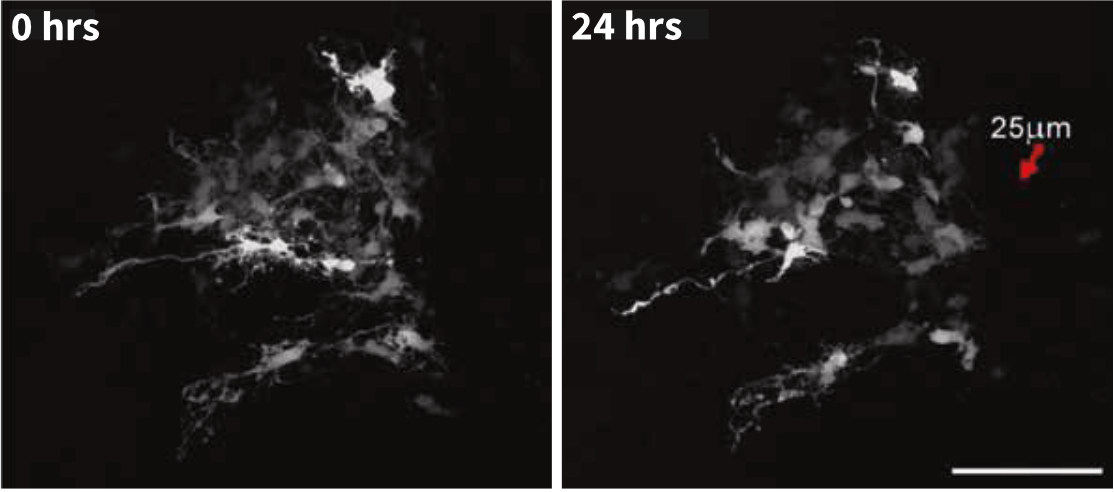
Above: Time lapse imaging of spinal cord tissue slice maintained on Alvetex Strata demonstrates minimal tissue drift over a period of 24 hours.
(Images courtesy of Kieran McDermott, University of Cork.)
Resources on explanting cells directly onto Alvetex
Whitepaper: Propagation of Human Pluripotent Stem Cells in Three-Dimensional Culture Using Alvetex Strata
Transfection
Various types of cells can be transfected using Alvetex 3D cell culture.
In collaboration with Mirus Bio, methods have been developed that enable the transfection of cells grown in Alvetex 3D culture. Common cell types (CHO-K1, HeLa, HepG2, MCF-7 and NIH-3T3) were seeded at optimized cell densities in 12 well Alvetex Scaffold 3D plates and adapted to 3D culture conditions for 48 hours. After adaptation, cells were transfected with a novel Mirus Bio formulation combined with a plasmid encoding firefly luciferase at the reagent-to-DNA ratios indicated beneath the bars. Luciferase activity was measured 24 hours post-transfection using a conventional assay. High expression was detected in all cell types demonstrating the efficiency of the Mirus Bio TransIT® 3D Transfection Reagent (MIR 5804) when used with Alvetex Scaffold 3D culture plates.
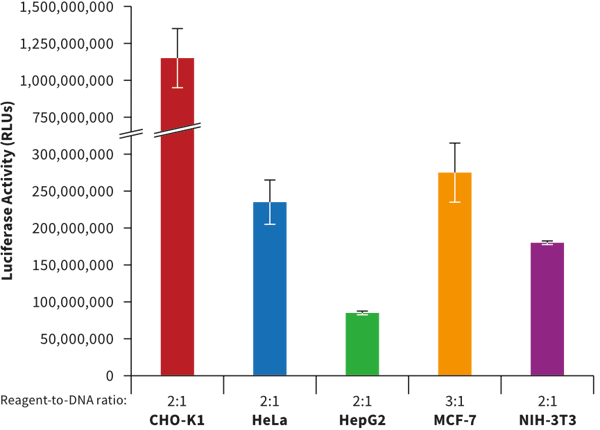
3D transfection of multiple cell types.
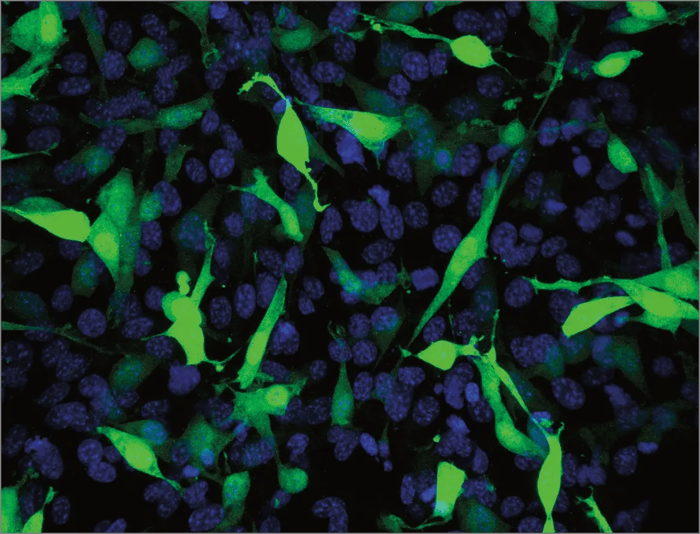
Above: Fibroblasts grown in 3D using Alvetex Scaffold were successfully transfected with a GFP construct and imaged using confocal microscopy. In brief, cells were transfected with the new Mirus Bio Transfection Reagent for 3D transfection at a reagent-to-DNA ratio of 3:1 using a GFP-expressing plasmid. Cells were seeded at 48 hours prior to transfection, and the cultures were fixed 24 hours post-transfection. Cells were imaged using a confocal microscope (Zeiss LSM510). The data shows a 40 μm integrated stack of multiple images as viewed from above the intact 3D culture. The position of all the cell nuclei are visualized with Hoechst 33342 (blue) and the positively transfected cells express GFP (green).
Resources on transfection of cells in Alvetex
Protocol: 3D Transfection System (Mirus)
Co-culture
Advancing from single cell mono-cultures and co-cultures in conventional 2D models, Alvetex Scaffold provides the next step towards replicating the in vivo environment by providing the architecture necessary for 3D cell culture in vitro. Alvetex’s extremely high porosity allows cells to penetrate, grow and proliferate throughout the material for highly effective and reproducible 3D cell culture. Cells are freely able to form complex interactions with adjacent cells and receive and transmit signals, enabling a more natural environment to foster the native architecture found in tissues.
As well as being able to study single cultures in 3D, Alvetex Scaffold provides a support that enables the co-culture of more than one cell type. The structure of tissues is often comprised of discrete layers of distinct cell types. Growing different cell types in 3D, inside and on the surface of Alvetex Scaffold, enables users to re-create such tissue structures in vitro. A variety of cell co-culture scenarios can be set up to study different cell-cell interactions, according to the requirements of the cells and the dynamics under investigation.
Key benefits and applications:
- Study interactions between distinct cell types in 3D culture
- Recreate in vivo tissue morphology
- Recreate specific niche environments for disease modelling or drug testing
- Customize co-culture setup to suit the cell types involved
Several alternative approaches for co-culture design can be utilised. Alvetex Scaffold is a versatile technology that enables users to create co-culture models in many different ways.
Assembly of Co-culturing Experiments
Key to image parts:
Alvetex Scaffold
in standard multiwell plate
![]()
Simplified diagram:
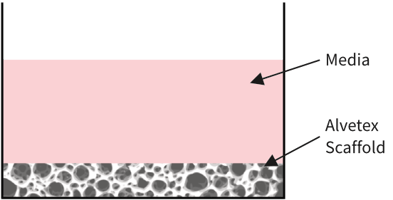
Alvetex Scaffold in well insert
in standard multiwell plate
![]()
Simplified diagram:
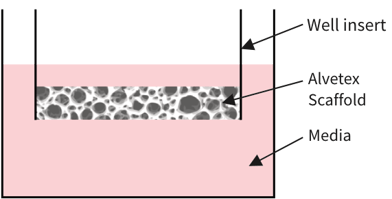

Alvetex Scaffold

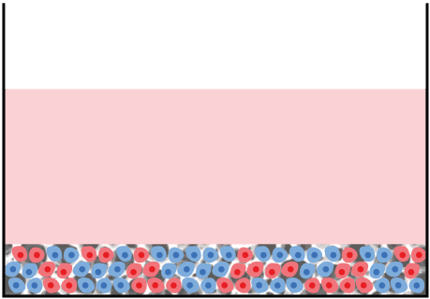
Cell type A growing in 3D within Alvetex Scaffold

Cell type B growing in 3D within Alvetex Scaffold

Mix of cells A & B in 3D within Alvetex Scaffold
![]()
Cells growing in 2D (monolayer)
Assembly option 1 – 3D co-culture in multiwell plate or well insert:
Description: Different cell types cultured together within the same scaffold.
Application: Emulate the structure of a tissue comprised of more than one cell type.
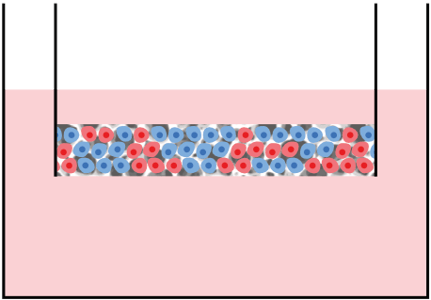
Assembly option 2 ‐ 3D / 2D co-culture in multiwell plate and well insert combined:
Description: Different cell types cultured together within the same scaffold within a well insert.
Application: Approach used to study the secretion of factors and signaling molecules.

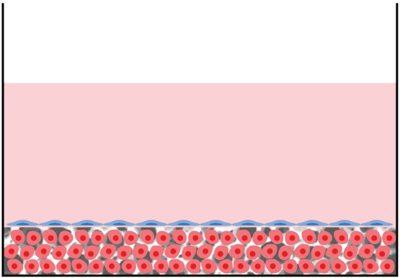
Assembly option 3 ‐ 3D co-culture in multiwell plate and well insert combined:
Description: Two independent 3D cultures. Contact is via medium – communication via paracrine factors.
Application: Approach used to study the secretion of factors and signaling molecules.
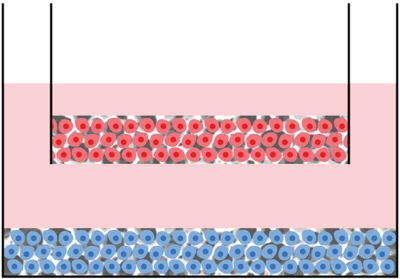
Assembly option 4 ‐ 3D / 3D co-culture in multiwell plate:
Description: Two 3D cultures in direct contact with one another.
Application:
- Study the direct interaction of cells in contact with one another.
- To establish layers of alternate cell types in 3D to mimic tissue structures.
- To investigate invasion and migration of different cell types amongst each other.
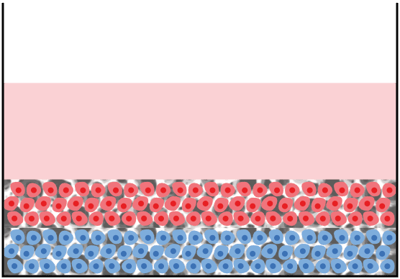
Assembly option 5 ‐ 2D / 3D co-culture in multiwell plate:
Description: One 2D (monolayer) and one 3D culture layered in direct contact with one another
Application:
- Study the direct interaction of cells in contact with one another
- To establish layers of alternate cell types in 3D to mimic tissue structures
- To investigate invasion and migration of different cell types amongst each other
Exmples of Co-culturing Experiments
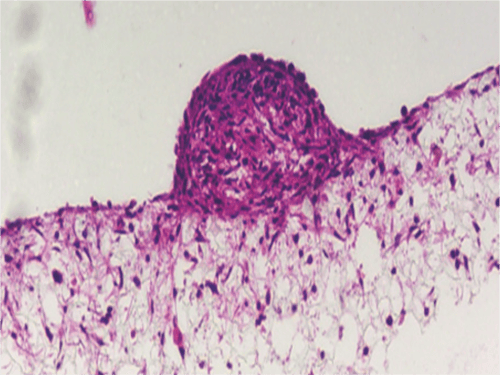
Above: Co-culture of glial and neural cells to model brain tissues. Brightfield micrograph showing the structure of a human stem cell-derived neurosphere co-cultured for 7 days with U118-MG glial cells on Alvetex Scaffold presented in the 12-well insert in 12-well plate format.
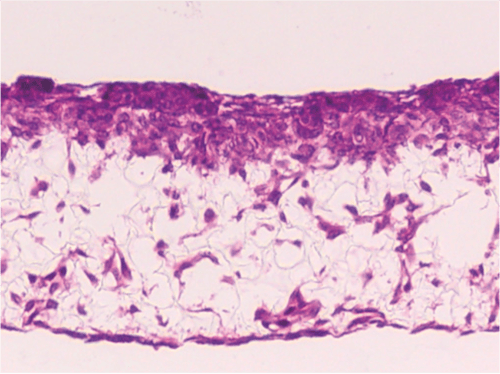
Above: Cell invasion into Alvetex Scaffold. Brightfield micrograph showing the structure of SW480 colon adenocarcinoma cells co-cultured for 7 days with established 3D cultures of 3T3 fibroblasts on Alvetex Scaffold.
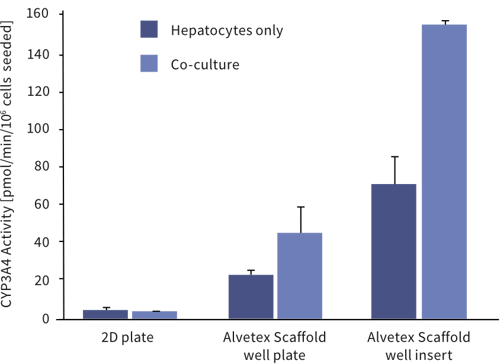
Above: Enhanced cell function with hepatocyte and endothelial cell co-culture. The activity of CYP3A4 in upcyte® hepatocytes cultured on a 2D plate and on Alvetex Scaffold presented in both a 12-well plate and 6-well insert formats. Hepatocytes were grown as mono-cultures and also co-cultured with upcyte® micro-vascular endothelial cells for 10 days. For further details please visit www.medicyte.com.
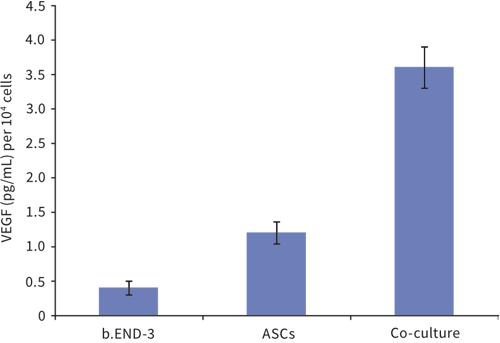
Above: Co-culture of adipose tissue-derived stem cells with endothelial cells influences their differentiation. Adipose tissue-derived stem cells (ASCs) and endothelial cells (b.END-3) were cultured independently and as co-cultures in Alvetex Scaffold 12 well plate format for 3 days. Data from 3 sample replicates. VEGF levels normalized to the number of cells at the time of seeding, expressed as pg/mL per 10 cells. For further details please refer to Neofytou, E.A., et al. Adipose tissue-derived stem cells display a proangiogenic phenotype on 3D scaffolds. J Biomed Mater Res A. 2011; 98(3): 383-93.
Resources on co-culturing cells in Alvetex
Protocols:
- Example protocol for the co-culture of the Caco-2 cell line with the CCD-18co cell line on Alvetex Scaffold in well insert formats
- Example protocols for the co-culture of either the SW620 or the SW480 cell line with the 3T3 cell line on Alvetex Scaffold in well insert formats
Some scientific papers featuring Alvetex co-cultures:
- In vitro toxicity of glyphosate in Atlantic salmon evaluated with a 3D hepatocyte-kidney co-culture model
L.Søfteland and P.A.Olsvik
Food and Chemical Toxicology, Volume 164. 14 April 2022. DOI: 10.1016/j.fct.2022.113012 - Bioengineering Novel in vitro Co-culture Models That Represent the Human Intestinal Mucosa With Improved Caco-2 Structure and Barrier Function
Nicole J Darling, Claire L Mobbs, Ariana L González-Hau, Matthew Freer and Stefan Przyborski
Frontiers in Bioengineering and Biotechnology. 31 August 2020. DOI: 10.3389/fbioe.2020.00992 - Characterization of liver specific functions of upcyte® hepatocytes grown in 3D and co-cultured with upcyte® endothelial cells
Dähn C, Hewitt N, Maltman D, Talas G, Przyborski S, Heinz S, Nörenberg A, Scheller K, Braspenning J
Z Gastroenterol, 2012; 50 - P2_05. 2012. DOI: 10.1055/s-0031-1295802
Alvetex Compatibility with Downstream Applications
Alvetex is compatible with a wide range of downstream applications, such as:
- Tissue processing, fixation, embedding and sectioning
- Brightfield microscopy and photographic imaging
- Cryostat sectioning
- Fluorescence microscopy, confocal, laser capture
- Flow cytometry and cytospinning
- Biochemical assays
- Histological staining, in situ hybridization
- Electron microscopy – both SEM and TEM
- Immunocytochemistry
- Isolation of viable cells
- Extraction of nucleic acid and total protein
Sectioning and Counterstaining
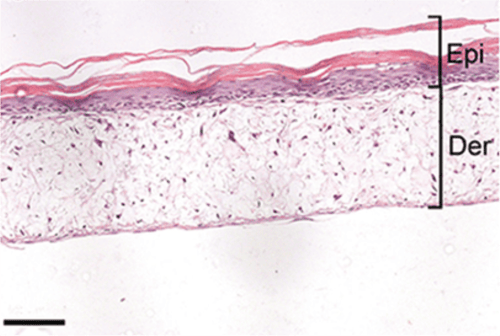
Unlike other 3D cell culture supports, Alvetex can easily be processed like a standard tissue sample. Frozen and paraffin embedded samples can be sectioned and stained to reveal the native cellular structures inside Alvetex. In this example, cells have been fixed in 4 % paraformaldehyde, embedded in paraffin wax, sectioned (7 μm) before staining with H&E and cover-slipped.
Resources on sectioning and counterstaining in Alvetex
Protocols:
- Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures
- Histology (2): Processing Alvetex Scaffold 3D Cultures for PFA Fixation and Paraffin Wax Embedding for Immunofluorescence
- Histology (3): Processing Alvetex Scaffold 3D Cultures for Bouin’s Fixation and Paraffin Wax Embedding for Haematoxylin and Eosin Staining
- Histology (4): Processing Alvetex Scaffold 3D Cultures for Resin Embedding and Toluidine Blue Staining for Light Microscopy
- Histology (5): Processing Alvetex Scaffold 3D Cultures for Scanning Electron Microscopy (SEM)
Immunocytochemistry
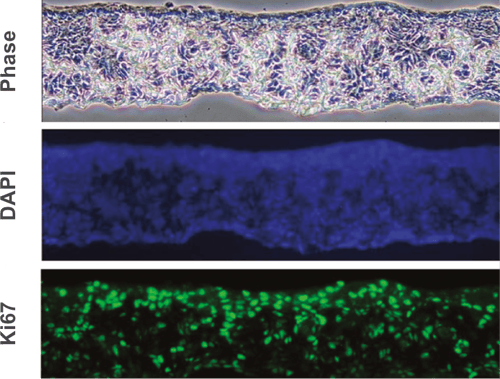
Ki67 DAPI Phase Immunocytochemistry used to visualize the expression of specific protein markers. In this example, cell cultures have been fixed in 4 % paraformaldehyde, embedded in paraffin wax and sectioned (10 μm). Antigen retrieval followed by immunocytochemical analysis with the proliferation marker Ki67 (green) and the nuclear stain DAPI (blue) was performed following standard immunocytochemical methods.
Resources on Alvetex and immunocytochemistry
Protocols:
Scanning Electron Microscopy

Visualization of the structure of 3D cultures in Alvetex Scaffold is made possible using scanning electron microscopy (SEM). Samples are prepared in the same manner as would normally be used for tissues. In this example, skin, cells have penetrated throughout the scaffold and some have stratified on the surface (lower right corner).
Resources on Alvetex and electron microscopy
Protocol: Histology (5): Processing Alvetex® Scaffold 3D Cultures for Scanning Electron Microscopy (SEM)
Whitepaper: A Review of Imaging Techniques Compatible with Three Dimensional Culture of Cells Grown in Alvetex Scaffold
Transmission Electron Microscopy

The ultrastructure of cells grown in Alvetex Strata can be analyzed by standard transmission electron microscopy (TEM). At high magnification, cellular structures such as these specialized bile canalicular cell protrusions are readily visualized.
Resources on Alvetex and electron microscopy
Protocol: Histology (5): Processing Alvetex® Scaffold 3D Cultures for Scanning Electron Microscopy (SEM)
Whitepaper: A Review of Imaging Techniques Compatible with Three Dimensional Culture of Cells Grown in Alvetex Scaffold
Gene Expression Analysis
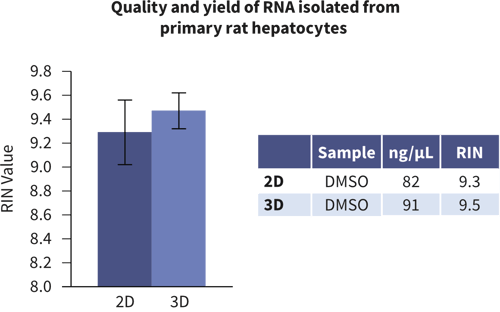
Isolation of nucleic acid from rat primary hepatocytes grown in Alvetex and conventional 2D cultures. RNA quality was determined by the RIN (RNA Integrity Number) and showed that the quantity and quality of RNA isolated from cells grown on Alvetex Scaffold was the same if not better than that isolated from standard 2D cultures. Data generated in collaboration with LGC (unpublished).
Resources on gene expression in Alvetex
Protocols:
- Extraction of RNA from Cells Cultured in Alvetex® Scaffold in 3D
- Extraction of RNA from Cells Cultured in Alvetex® Scaffold in 96-Well Plate Format
Application note: Three-Dimensional Culture of HaCaT Keratinocytes Using Alvetex Scaffold
Protein Expression Analysis
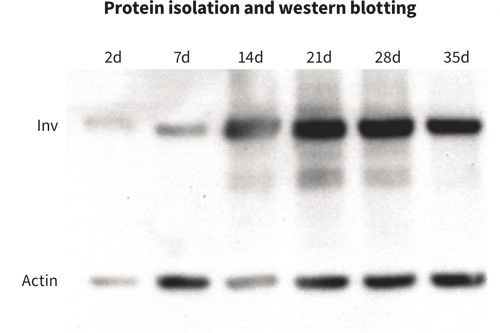
With standard lysis protocols, total protein can be efficiently isolated from cells growing inside Alvetex. This allows for more biologically relevant protein expression analysis experiments to be carried out. Here we showed the increase over time of involucrin (Inv) expression in maturing keratinocytes by western blot analysis.
Resources on protein expression in Alvetex
Protocols:
- Total Protein Extraction from Cells Cultured in Alvetex® Scaffold in 3D
- Protein Extraction from Cells Cultured in Alvetex® Scaffold 96-Well Plate Format
Application notes:
Isolation of Cells from Alvetex
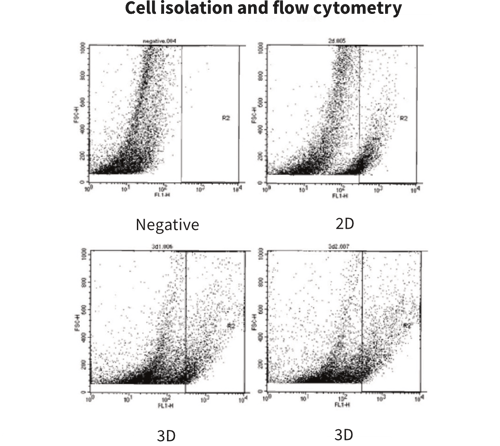
With standard lysis protocols, total protein can be efficiently isolated from cells growing inside Alvetex. This allows for more biologically relevant protein expression analysis experiments to be carried out. Here we showed the increase over time of involucrin (Inv) expression in maturing keratinocytes by western blot analysis.
Resources on isolation of cells from Alvetex
Protocols:
Biochemical Assays
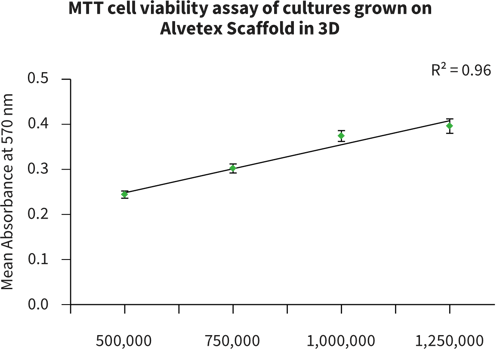
Cells growing in 3D inside Alvetex can be studied using typical biochemical assays such as cell viability assays, apoptosis assays, cell proliferation assays etc. Here we show the measurement of cell viability using a standard MTT assay.
Resources on metabolic assays in Alvetex
Protocols:
- alamarBlue® cell viability assay of upcyte® hepatocyte cultures grown on Alvetex Scaffold in 3D
- Determination of cell number and growth characteristics within Alvetex Scaffold using the PicoGreen® assay
- XTT cell viability assay for Alvetex Scaffold 96 well plate format
- MTS cell viability assay of upcyte® hepatocyte cultures grown on Alvetex Scaffold in 3D
- MTT cell viability assay of cultures grown on Alvetex Scaffold in 3D
Perfused 3D Cell Culture
Alvetex perfusion plates enable dynamic circulation of culture media through wells into which Alvetex well inserts can be suspended. These systems can be used to create complex co-cultures, multi organ systems and to study paracrine effects.

Above. A: Alvetex Scaffold (scanning electron micrograph image) B: Alvetex Scaffold 6 well inserts. C: Cells grown in Alvetex maintain their 3D structure and form interactions with adjacent cells, resulting in a tissue-like structure. D: Alvetex well inserts fit inside the perfusion plate wells, enabling the flow of culture medium above and below the 3D culture.
Resources on Alvetex perfusion plates
Application note: Alvetex Perfusion Plate: Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate
Advanced 3D Tissue Bioengineering
The Alvetex Advanced 3D Tissue Bioengineering System is designed for scientists who need biologically relevant, reproducible models such as skin for testing pharmaceuticals , cosmetics , chemicals , and devices .
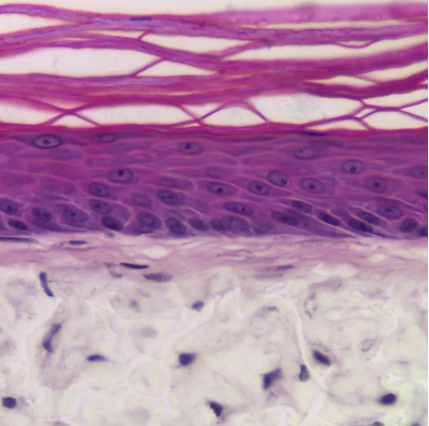
Above: Bioengineered full thickness human skin model (sample prepared for histology and stained with H&E). The white structures within the dermal compartment are Alvetex Scaffold.
See also REPROCELL's Alvetex 3D Bioengineered Tissue Model Assay Services.
Resources on the Alvetex Advanced 3D Tissue Bioengineering System
Application note: Alvetex Perfusion Plate: Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate
Alvetex Plasticware Ranges
There are currently two ranges of Alvetex plasticware (bespoke plasticware containing Alvetex membrane).
Alvetex 3D Cell Culture Systems:
- A platform for routine 3D cell culture, designed for cell biologists who want to move beyond standard 2D monolayer cell cultures to 3D.
- Designed for routine mammalian and human 3D cell culture and basic co-culture models.
- Also suited for labs transitioning to 3D without full tissue engineering complexity.
Alvetex Advanced 3D Tissue Bioengineering System:
- Advanced platform for 3D tissue bioengineering with multiple cell types.
- Designed for building organotypic tissue models (e.g., skin, mucosa, barrier tissue).
- Designed for Pharmaceutical / cosmetic R&D teams that need reproducible models for barrier‐function measurements (TEER, water loss, permeability), topical application, and device/tissue interface studies.
Comparing Alvetex Systems
| Features | Alvetex 3D Cell Culture Systems | Alvetex Advanced Tissue Bioengineering System |
|---|---|---|
| Summary | Alvetex membrane (polystyrene scaffold, 200 µm thick, >90% porosity) presented in multi well culture plates or bespoke inserts | Alvetex membrane (polystyrene scaffold, 200 µm thick, >90% porosity) integrated in a more complex insert with modular handles and deep (high capacity) media plates |
| Purpose | Routine 3D cell culture (mono- and co-culture) | Advanced 3D tissue bioengineering |
| Primary applications | Basic 3D cell culture, co-culture, functional studies, assays | Tissue engineering (skin, mucosa, barrier tissue), device & cosmetic testing, complex in vitro tissue disease models |
| Testing/ assays (examples) | MTS, MTT, XTT | TEER, clinical devices, skin barrier permeability |
| Target audience(s) | Academic labs, cell biologists, toxicity/ metabolism labs, stem cell/ tumor labs. | Translational R&D, pharma, cosmetic industry, tissue‐engineering labs, high‐throughput barrier assay users. |
| Alvetex membrane | Alvetex Scaffold (in plates and inserts), and Alvetex Strata (in inserts). Mammalian cells grow within Alvetex Scaffold or on the surface of Alvetex strata. | Alvetex Scaffold only. |
| Cell morphology and function | Cells maintain more in vivo-like morphology, with improved viability and function compared to 2D cell culture. | Applies these benefits into tissue models. |
| Plasticware formats |
|
Advanced 15mm modular inserts, requiring holder or handles (low, medium, high) for supporting over bespoke deep 6-well or single well plates. |
| Auxiliary plasticware |
|
Advanced deep 6 well plates and (shared high capacity) single well plates can have uses beyond their original purpose within the Alvetex Advanced Tissue Bioengineering System. |
Alvetex 3D Cell Culture Systems
Alvetex 3D Cell Culture Systems are built around Alvetex, the highly-porous polystyrene scaffold (200 µm thick, >90 % porosity). Alvetex products come in gamma sterilized packs with a very long shelf life — as long as the sterile pack remains unopened.
Alvetex 3D Cell Culture Systems have been designed to make 3D cell culture routine in the laboratory, and easy to migrate to for scientists who are familiar with conventional 2D culture. Alvetex is compatible with the same plasticware, same culture media, same cell visualisation techniques, same cell based assays, and same downstream applications — with the results being much more comparable to cells grown in 3D in vivo — cells grown within Alvetex Scaffold or on top of Alvetex Strata maintain a true 3D architecture rather than deform and flatten onto a 2D surface.
Typical applications of Alvetex 3D Cell Culture Systems include basic cell biology, drug metabolism/toxicity screening, stem cell differentiation, tumour‐cell biology in 3D, and simple co-culture models.



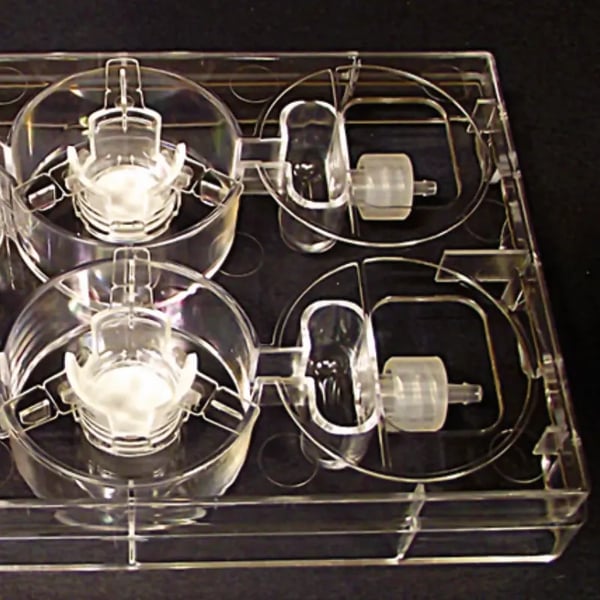
Above: A. Alvetex Scaffold 12 well plate (AVP002). B. Alvetex Scaffold 6 well inserts (AVP004). C. Alvetex Well Insert Holder (AVP015). D. Alvetex Perfusion Plate (AVP011).
| Product Name | Product code |
|---|---|
| Alvetex Scaffold Multiwell Plate Formats — packs of 2, 10 or 80 | |
| Alvetex Scaffold 12 Well Plate | AVP002 |
| Alvetex Scaffold 24 Well Plate | AVP006 |
| Alvetex Scaffold 96 Well Plate | AVP009 |
| Alvetex Scaffold Well Insert Formats — packs of 12, 48 or 96 | |
| Alvetex Scaffold 6 Well Insert | AVP004 |
| Alvetex Scaffold 12 Well Insert | AVP005 |
| Alvetex Scaffold 24 well insert | AVP012 |
| Alvetex Strata Well Insert Formats — packs of 12, 48 or 96 | |
| Alvetex Strata 6 Well Insert | STP004 |
| Alvetex Strata 12 Well Insert | STP005 |
| Alvetex Tools — packs of 2 or 10 | |
| Alvetex Well Insert holder and Deep Petri Dish | AVP015 |
| Alvetex Perfusion Plates with Luer Locks | AVP011 |
| Alvetex Kits | |
| Alvetex Scaffold Plate Starter Kit 1 × 12 well plate / 1 × 24 well plate / 1 × 96 well plate |
AVP-KIT-1 |
| Alvetex Scaffold Well Insert Starter Kit 6 × 6 well inserts / 6 × 12 well inserts / 1 × well insert holder and deep Petri dish |
AVP-KIT-2 |
| Alvetex Strata Well Insert Starter Kit 6 × 6 well inserts / 6 × 12 well inserts / 1 × well insert holder and deep Petri dish |
STP-KIT-2 |
| Perfusion Plate Kit with Alvetex Scaffold 6 well inserts 2 × Perfusion Plates / 12 × Alvetex Scaffold 6 well inserts |
AVP-KIT-3 |
| Perfusion Plate Kit with Alvetex Scaffold 12 well inserts 2 × Perfusion Plates / 12 × Alvetex Scaffold 12 well inserts |
AVP-KIT-4 |
| Perfusion Plate Kit with Alvetex Scaffold 6 well inserts 5 × Perfusion Plates / 48 × Alvetex Scaffold 6 well inserts |
AVP-KIT-5 |
| Perfusion Plate Kit with Alvetex Scaffold 12 well inserts 5 × Perfusion Plates / 48 × Alvetex Scaffold 12 well inserts |
AVP-KIT-6 |
Note: all products are supplied in sterile gamma irradiated packs.
▶ Discover more: Featured Products: Alvetex 3D Cell Culture Systems
Alvetex Advanced Tissue Bioengineering System
Built on the same Alvetex scaffold technology, the Alvetex Advanced Tissue Bioengineering System is REPROCELL’s next-generation 3D tissue platform. Alvetex Advanced is designed for:
- Scientists working in tissue engineering and translational researchers building organotypic tissue models (e.g., skin, mucosa, barrier tissue).
- Pharmaceutical and cosmetic R&D can create reproducible models for studying skin barrier‐function (e.g. TEWL (transepidermal water loss), TEER (transepithelial electrical resistance), permeability), topical application, burn and wound healing, etc.
- Paracrine/crosstalk modelling can also be studied where multiple tissue models are submerged and sharing the same culture medium.
The key component is the the Alvetex Advanced 15 mm Modular Insert (AVP022), that holds a 15 mm disc of Alvetex Scaffold with a leak-free tight seal around its circumference. It is modular and highly versatile, in that it fits with either the well insert holder (included with AVP022 or separately as AVP030) one of three bespoke handles that hold the inserts at different heights (low, medium, or high) within the large media capacity deep plates of the Alvetex Advanced Tissue Bioengineering System.

Above: Alvetex Advanced 15 mm modular inserts (AVP022).
All components of the Alvetex Advanced Tissue Bioengineering System come in gamma sterilized packs with a very long shelf life — as long as the sterile pack remains unopened.




Above: A. Alvetex Advanced 15 mm modular inserts with insert holder (AVP022). B. Low (AVP024), medium (AVP025), and high (AVP026) insert holders. C. Advanced deep 6 well plates (AVP026). D. Advanced single well plate, 125 ml (AVP029).
| Product name | Product code |
|---|---|
| Alvetex Advanced Modular Inserts — packs of 6, 12, 24, 48, 60, or 96 inserts | |
| Alvetex Advanced 15mm Modular Inserts containing Alvetex Scaffold membrane In blister packed units of 6 inserts in an insert holder, with lid |
AVP022 |
| Handles for Alvetex Advanced modular inserts — packs of 12, 24, 48, 60, or 96 handles | |
| Low handles | AVP023 |
| Medium handles | AVP024 |
| High handles | AVP025 |
| Advanced Plates — packs of 5, 10 or 15 plates with lids | |
| Advanced deep 6 well plates | AVP026 |
| Advanced single well plates, 50 ml | AVP027 |
| Advanced single well plates, 75 ml | AVP028 |
| Advanced single well plates, 125 ml | AVP029 |
| Handle holders and insert holders | |
| 6-Well Handle Holders for Use With Advanced Single Well Plates | AVP030 |
| 6-Well Holders for Alvetex® Advanced Modular Inserts, With Lid | AVP031 |
| Alvetex Advanced Kits | |
| Alvetex Advanced Starter Kit – 15mm Modular Inserts with Adjustable Heights | ADV-KIT-1 |
| Alvetex® Advanced Starter Kit – Co-culture and Paracrine Study Platform | ADV-KIT-2 |
Note: all products are supplied in sterile gamma irradiated packs.
▶ Discover more: Featured Products: Alvetex Advanced Tissue Bioengineering System
Alvetex 3D Bioengineered Tissue Model Assay Services
REPROCELL's 3D bioengineered human tissues, produced using Alvetex, complement our fresh tissue assays by accurately recapitulating human biology in longer duration experiments, allowing investigations into mechanisms that are key to many drug targets including fibrosis, wound healing and epithelial barrier disruption.
Our human tissue models are also useful for testing other substances including chemicals, consumer products, cosmetics and pollutants.
IPF Model
Our idiopathic pulmonary fibrosis (IPF) model is the most effective model available for investigation of drugs to treat pulmonary fibrosis. The model uses human primary lung fibroblasts from patients with IPF and an alveolar type II cell line to create a 3D model of the lung. This is the most sophisticated in vitro tissue modelling fibrotic lung.
Skin Model: REPROSKIN™
Our cutting-edge full-thickness engineered skin model, constructed from human primary cells, represents the forefront of the market for drug discovery assays. This model consists of a dermal layer supporting a stratified keratinized epithelial layer, providing a highly accurate representation of human skin. Our comprehensive skin model offers a variety of investigative services tailored to meet the diverse needs of drug discovery research.
Neurite Outgrowth Model
Measuring neurite outgrowth allows for the evaluation of the impact of test drugs on neurite formation or toxicity. Our translational neurite outgrowth model integrates Alvetex scaffolds with iPSC-derived human neurons, resulting in the formation of a mature neuronal network complete with synaptic connections. This innovative model provides a comprehensive platform for assessing drug effects on neuronal function and viability.
▶ Discover more: Bioengineered 3D Tissue Model Services