PBMC processing and isolation
Obtain mononuclear cells with high viability and purity
Our scientists can process your blood samples to generate mononuclear cells with high viability and purity. Working to GCLP standards, we can derive high-quality PBMCs using low-ionic-strength diluents and density gradient centrifugation.
We can work with a range of blood sample types to derive PBMCs, including:
- Whole blood samples
- Buffy coat samples
- Leukopaks
Plasma or serum fractions can also be collected, aliquoted, and stored to meet your specific requirements.
No matter what the starting material for your PBMCs, we have the best processing option available for your project.
Controlled rate PBMC freezing and storage
Maintenance of the cold chain is critical to PBMC quality, so the processing of your sample by our scientific team is temperature-controlled throughout.
For optimal cell viability and function, your sample will be:
- processed upon arrival in our BSL-2 lab (usually within 4 hours)
- frozen via controlled-rate freezing and using a low-endotoxin, serum-free, and xeno-free cryopreservation medium
- stored in a monitored freezer system at optimal temperature
Maintain chain of custody
To ensure chain of custody, your PBMC processing project is supported by our GLP- and GCLP-compliant labs, rigorous SOPs, and highly qualified staff. Our team's internal quality controls and protocols can be adjusted to match your clinical protocol specifications, ensuring the correct handling of your blood sample and PBMCs from project initiation to completion.
PBMC Processing Methodology
1
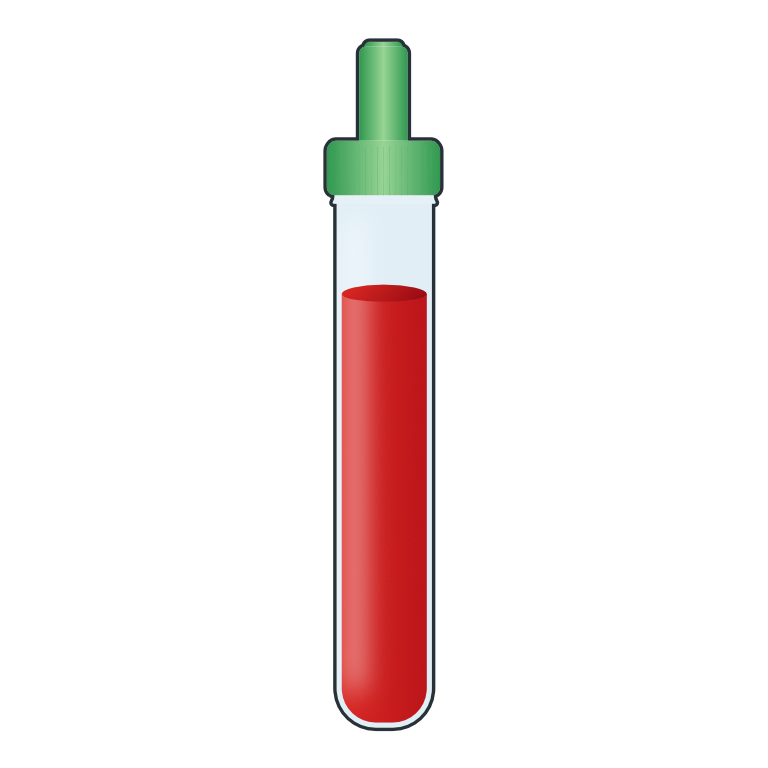
Whole blood sample
 Overlaying the diluted blood onto density gradient medium (DGM)
Overlaying the diluted blood onto density gradient medium (DGM)2
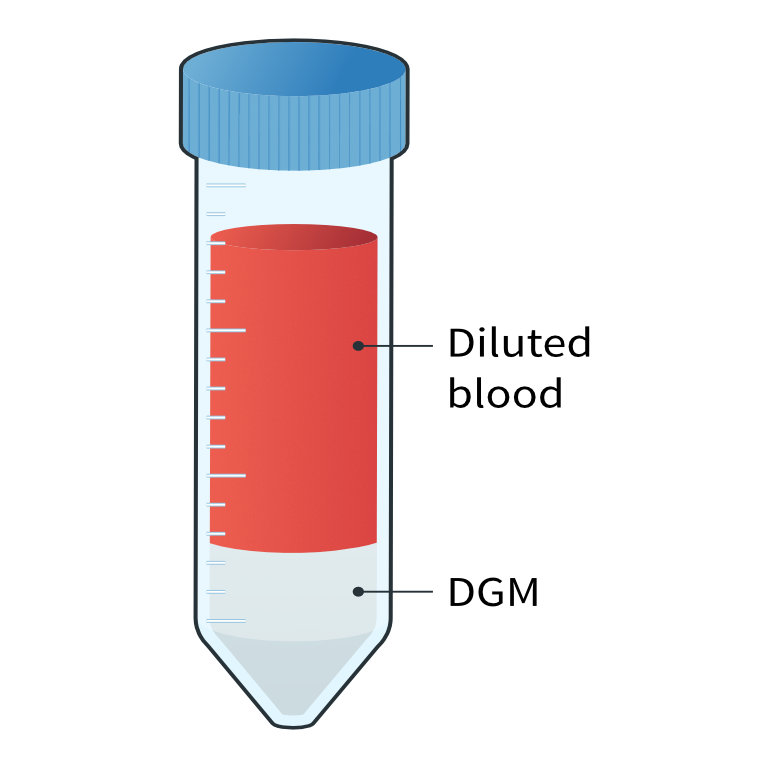
Diluted blood layered on density gradient medium
3
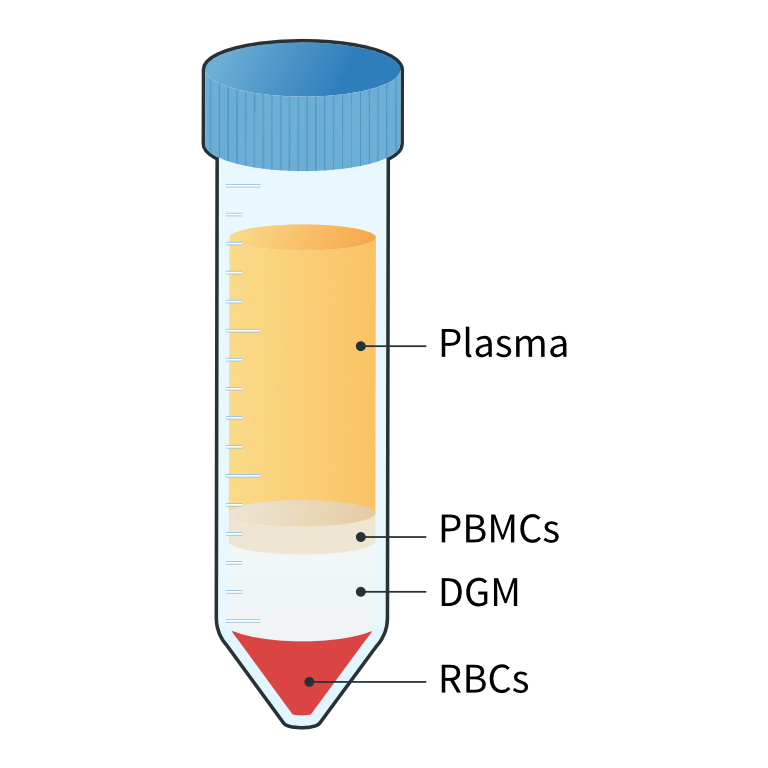
Separation of diluted blood into 4 distinct layers
 Collection of the ‘buffy coat layer’ with PBMCs
Collection of the ‘buffy coat layer’ with PBMCs4
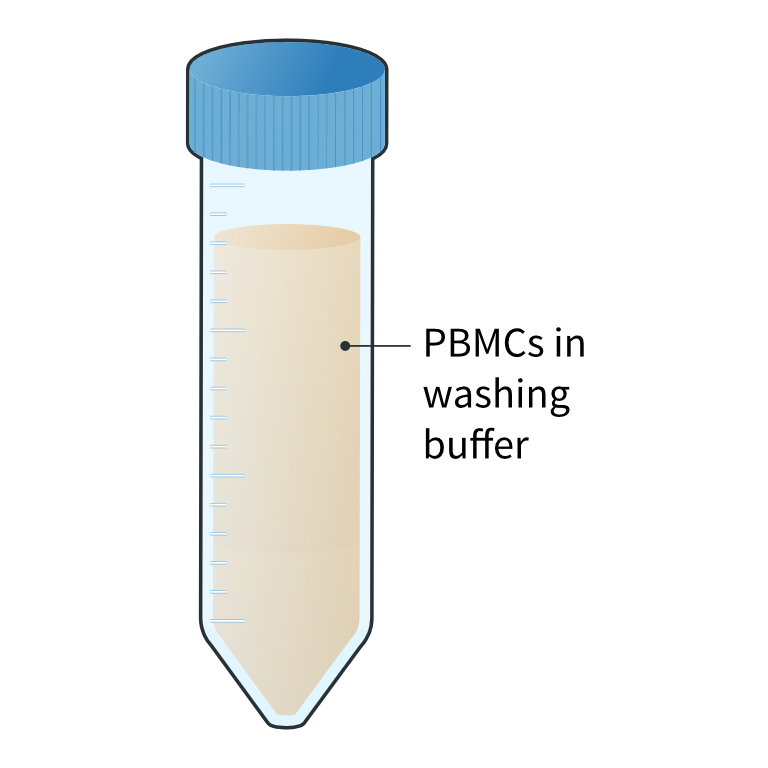
Preparation of PBMC suspension

5
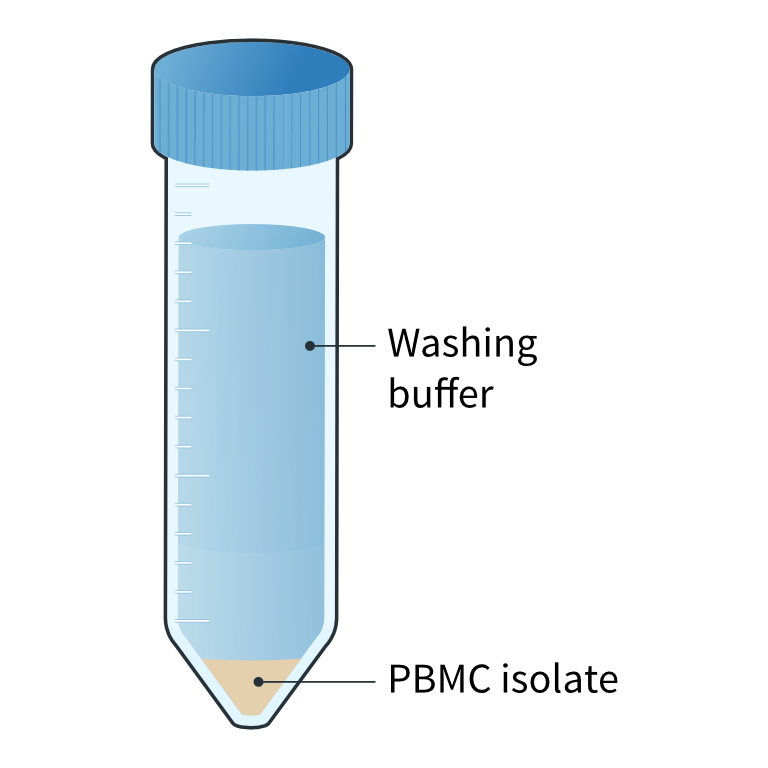
Washing of PBMC isolate
 Manual cell counting and calculation of the cell yield
Manual cell counting and calculation of the cell yield6
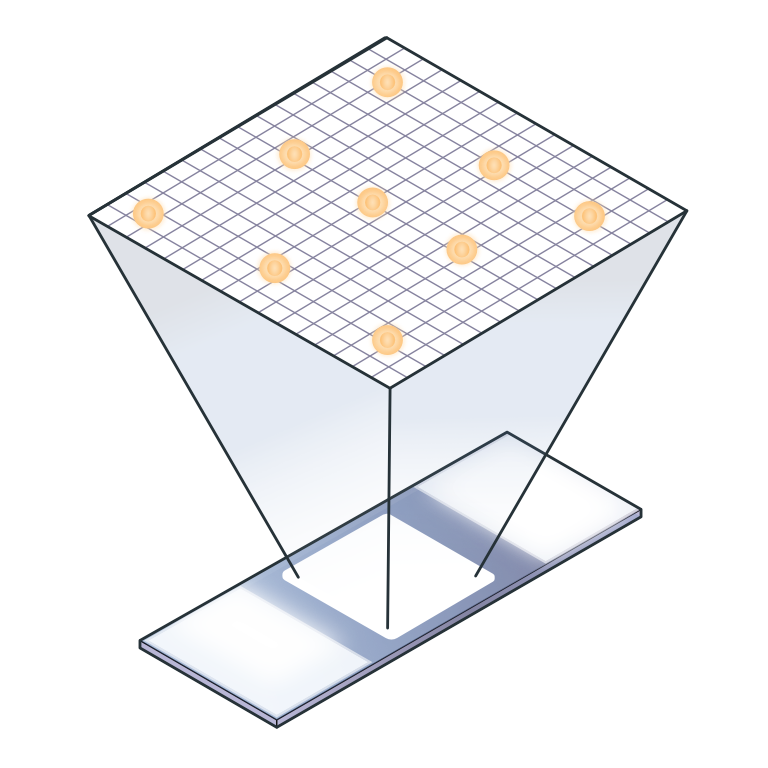
Total cell number, viability and PBMC yield records
 Controlled-rate PBMC freezing
Controlled-rate PBMC freezing7
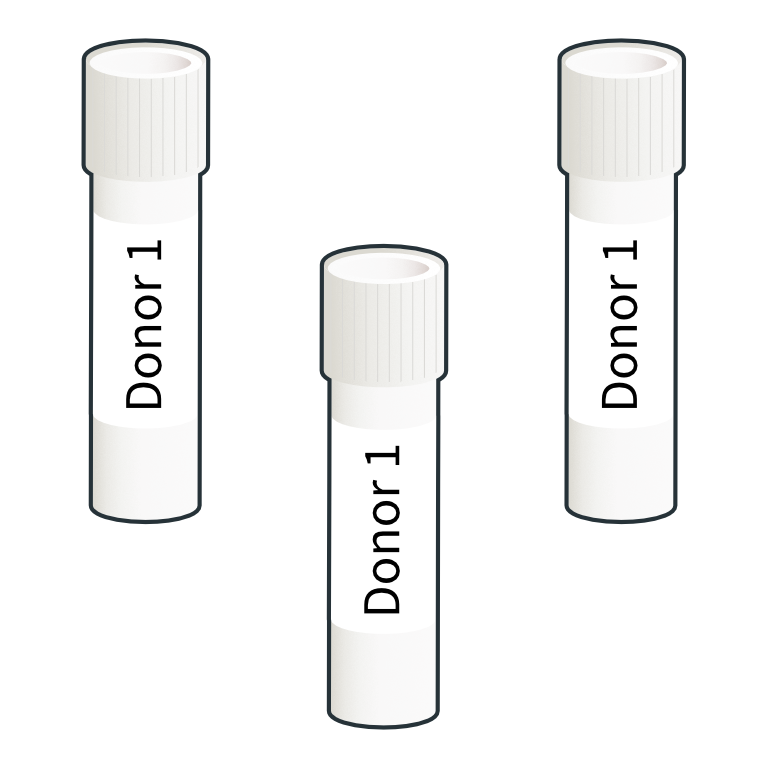
Labelling and cryopreservation
 Transferring of cryovials to liquid nitrogen (LN2)
Transferring of cryovials to liquid nitrogen (LN2)8
Long term cryostorage of PBMC lots
Frequently Asked Questions
We have listed some of the most frequently asked questions about our PMBC processing service below.
Q1. What type of blood samples can you isolate PBMCs from?
The most common source we work with is adult peripheral blood. Blood samples are collected in specialized tubes of varying volumes containing an anticoagulant such as K2EDTA or LiHep, and are usually processed within 24 hours of collection. The number of PBMCs per mililiter of blood can vary depending on the patient age and disease state e.g., pediatric patients have a higher concentration of PBMCs compared to adult populations. On average, we can isolate between 0.5-3x106 PBMCs/mL from an adult peripheral blood sample.
In addition, our team can isolate PBMCs from alternate blood products such as buffy coat and leukopaks. Buffy coat collections -a side product of blood donation - typically contain around 50 mLs of blood and 500 million PBMCs. Leukopaks are larger again with around 100 mLs of blood and generate approximately 5x107 PBMCs/mL.
Q2. Can you describe your typical PBMC processing workflow?
In-house or sponsor specific PBMC workflows can be followed. Generally, when working with whole blood samples, we dilute the blood and overlay it on a density gradient medium, such as Ficoll-Paque or Histopaque. We then perform centrifugation with a centrifuge that allows temperature and brake adjustment. After centrifugation, distinct layers are visible in the tube; red blood cells and granulocytes are located below the PBMC fraction, and the plasma layer above. The layer containing PBMCs is then collected and subjected to a series of washes and centrifugations with a specific diluent prior to freezing.
Q3. What happens after PBMC processing?
To assess PBMC quality, we use manual (trypan blue exclusion) or automated cell counting to determine cell number and viability. PBMCs are resuspended in a cryoprotectant medium and aliquoted into cryovials at the desired concentration. Our team then use controlled rate freezing protocols to freeze your PBMC. Within 1-3 days, they are transferred into vapor phase liquid nitrogen for long-term cryogenic storage.
Q4. What reagents do you use for PBMC isolation?
Reagents can vary depending on sponsor specific protocols but generally we use the following to ensure optimal separation and isolation of your PBMC fraction:
- For separation - a density gradient medium - typically Ficoll - which has a density of 1.077 g/ml.
- For wash steps - a low ionic strength, calcium and magnesium free DPBS supplemented with BSA or FBS.
- For cryopreservation - a low endotoxin, culture grade Dimethyl Sulphoxide (DMSO) with animal or human serum or a commercial cryopreservation media optimized for PBMC isolation.
Some serums and reagents may affect downstream applications, so we work with sponsors to identify the optimal reagents for their specific needs.
Q5. How do you assure quality in your PBMC isolation process?
We operate a GCLP quality system covering all aspects of our process to ensure quality for the process and the resultant material. Various factors can affect the quality of isolated PBMCs during processing - time to processing, temperature, reagents, freezing protocols etc. We take steps to ensure that all our processes are optimised and validated to ensure that PBMC quality is not compromised. We minimise transit time to the laboratory and maintain optimal transit temperature throughout, process samples upon arrival (within 24 hours of blood draw). and perform our isolation protocols efficiently within 4 hours. Freezing is gradually applied at a rate of ~1°C/min to prevent severe cell damage, osmotic injury, or the formation of intra- extra-cellular ice crystals. And all storage facilities are monitored.
Additionally, our certified staff undertake protocol-specific PBMC processing training (if required), we operate GLP- and GCLP-compliant labs, and implement rigorous SOPs and internal quality control systems.
Speak to our experts
At REPROCELL, we provide unparalleled genotyping, human tissue samples, and DNA extraction services. Do you have an inquiry about our services? Contact us today and we will be happy to answer.
.jpg?width=756&height=425&name=blog%20images%20(1).jpg)

