Example Preparation of HaCaT Epidermal Equivalent Using Alvetex® Scaffold
● Download this protocol as a PDF (5.7 MB)
1. Introduction
HaCaT is an immortalised human adult keratinocyte cell line. This cell line has been known to retain a capacity fornormal differentiation up to multiple passages similar to normal human epidermal keratinocytes (NHEK), and hence offers a suitable model for keratinisation studies. However, even with their highly preserved differentiation capacity, terminal differentiation can remain incomplete under air-exposed conditions. This protocol describes a method for differentiation of HaCaT cells under air-exposed conditions for up to 21 days.
2. Method
- HaCaT cells were routinely maintained in T-75 flasks in 2D using complete media (Dulbecco’s Modified Eagle Medium supplemented with 10 % v/v FBS, 2 mM L-glutamine and 100 U/mL Penicillin/ Streptomycin).
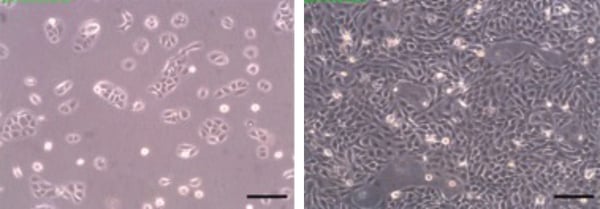
Figure 1. Phase contrast micrographs of HaCaT cells grown in conventional 2D culture flasks. Images show cells at low (left) and high (right) confluency. Scale bars: 200 µm.
- Cells were harvested by trypsinisation and centrifuged for 5 minutes (1000 rpm). The supernatant was discarded and the cell pellet re-suspended in an appropriate volume of media for dilution for cell counting by Trypan Blue stain.
- Cells were re-suspended at a concentration of 20 × 106 cells/mL in Clonetics® Keratinocyte Growth Medium-2 (KGM®-2; Lonza, CC-3107) for seeding.
- In the meantime Alvetex Scaffold 12-well inserts (AVP005) were prepared for seeding as described in the product information booklet. Briefly, well-inserts were dipped into a beaker containing 70 % ethanol before placing into 6-well plates after gentle tapping to release any excess ethanol. Well inserts were immediately washed with media (7 mL/well).
- The media was aspirated and replaced with a second media wash. This was aspirated just before application of the cells: 1 × 106 cells in 50 μL were added to the middle of each well insert.
- The plate was incubated for 30 minutes at 37 °C with 5 % CO2 to allow the cells to settle, wells were then carefully topped up with 10 ml of KGM®-2 and cultured for 3 days under submerged conditions. Culture media was fully changed after 2 days.
- Well inserts were then moved to REPROCELL’s custom-made Well insert holder in a deep Petri dish (AVP015) at medium setting, and maintained at the air/liquid interface (37 mL/per Petri dish) for up to 21 days to promote stratification. Culture media was refreshed by partial media exchange (50:50) every 2 days.
- Cultures were examined histologically at the end of each week. 3D cultures were processed in either Bouin’s fixative or 4 % paraformaldehyde, wax embedded, sectioned (10 μm) and analysed by haematoxylin and eosin staining or immunohistochemistry. For full experimental details please refer to specific histology protocols – see Alvetex Protocols.
3. Example Data Set
Between 7 and 14 days the HaCaT cells colonised 100 % of the Alvetex Scaffold disc (Figure 2), with the majority of cells still mitotic in nature as indicated by ki67 antibody staining (Figure 3). After 21 days, cell differentiation is noticeable within the construct, indicated by intense pink staining of the cell cytoplasm due to increased keratin production, anucleation and flattening of cell morphology. This is further supported by the expression of late differentiation markers (keratin 10 and involucrin) which occur towards the air exposed surface of the culture with increasing expression from 7-21 days (Figure 3).
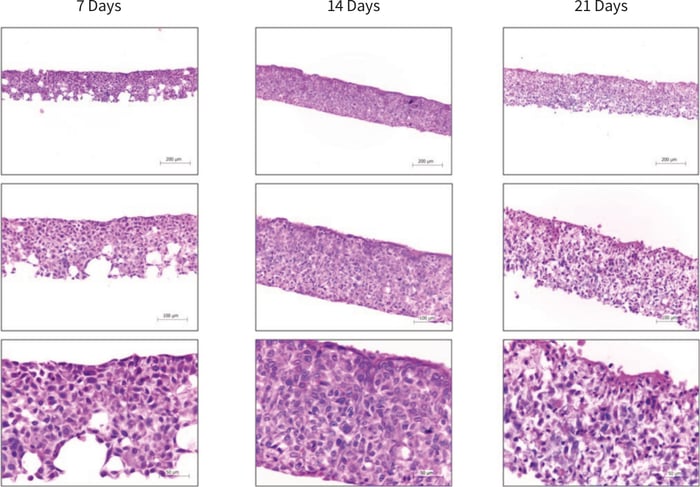
Figure 2. Brightfield micrographs showing the structure of HaCaT cells cultured on Alvetex Scaffold in 12-well inserts (AVP005) by haematoxylin and eosin staining of Bouin’s fixed, paraffin embedded sections. HaCaT cells were initially cultured for 3 days under submerged conditions prior to air exposure. The time-points referred to in the figures represent the number of days cultures subsequently spent at the air/liquid interface.
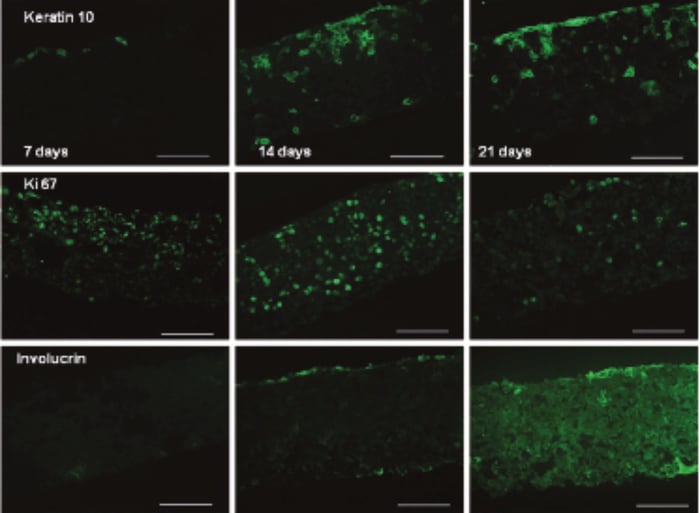
Figure 3. Keratin 10 (mouse monoclonal), Ki67 (rabbit polyclonal) and involucrin (mouse monoclonal) expression of HaCaT cells cultured on Alvetex Scaffold in 12-well inserts (AVP005) by immunohistochemical analysis of 4 % formaldehyde fixed, paraffin embedded sections (secondary antibodies, Alexa Fluor 488 donkey, anti-mouse and Alexa Fluor 488 goat, anti-rabbit). HaCaT cells were initially cultured for 3 days under submerged conditions prior to air exposure. The time points referred to in the figures represent the number of days cultures subsequently spent at the air/liquid interface (scale bars 100 µm).
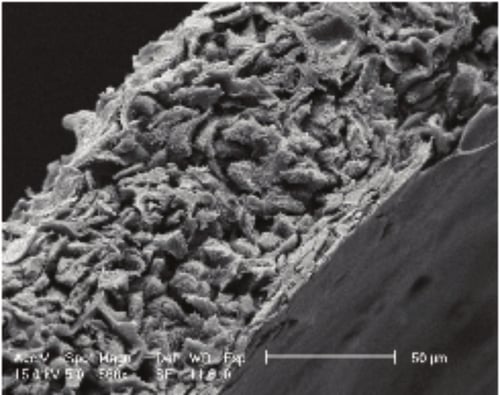
Figure 4. Scanning electron micrograph (SEM) image showing HaCaT cells grown in Alvetex Scaffold cultured for 7 days at the air/liquid interface (scale bar 50 microns).