CASE STUDY – Amgen
Avoid Species Differences: Rescue your drugs from clinical failure
Our case study on Amgen's publication highlights the useful comparisons that can be made between matched tissues from preclinical species and humans.
It is not uncommon that at a late stage of drug development (especially during clinical testing) unforeseen toxicities and/or poor efficacy are observed. This is often, but not only, due to the limitations of the off-target screening used by pharma (the so-called secondary pharmacology).
Although still useful for quickly screening a relatively high number of potential off-target hits, these screening studies, often used by pharmaceutical companies, are in the form of a proprietary set of in vitro methods biased and/or tailored for the specific molecule background of the drug candidate. Often, this does not allow evaluation in a clinical context and can contribute to the high rate of drug failures (attrition rate) at a later stage of the drug discovery process. This is a costly “Achilles heel” for the pharmaceutical industry.1
A recent publication2 highlights the importance of incorporating the use of fresh human tissue-based assays to better inform scientists and clinicians during drug development. The human/clinical context, that such assays offer, provides a powerful tool for a better understanding of off (and on) target activities which can only accelerate drug discovery and reduce the attrition rate even at a clinical stage.
Toxicity challenges faced during clinical trial
In the paper, the authors firstly showed how a drug candidate for solid tumors, AMG 337, was a highly selective inhibitor of the proto-oncogene mesenchymal-epithelial transition factor (c-Met).2 C-Met is a receptor tyrosine kinase also known as hepatocyte growth factor receptor (HGFR). Its gene is often amplified or mutated (activating mutations) in many solid tumors and plays key roles in cell proliferation, survival, invasion and tumor angiogenesis.3
Furthermore, its aberrant expression has been associated with drug-resistance in some tumors.4,5 In an off-target screening study, it was found that AMG 337 had only one off-target hit: the adenosine transporter (AT). Interestingly, a dose-limiting side effect (headache) was detected during clinical development.

Determining the cause of toxicity
Subsequently, the authors conducted a series of laboratory assays in an attempt to test the hypothesis of a causative link between the off-target inhibition of AT, extracellular accumulation of adenosine, subsequent adenosine-driven vasodilation via adenosine receptor (AR) and ultimately the high incidence of headaches at increasing doses of AMG 337.
Firstly, in vitro assays, assessing uptake of radioactive adenosine in the presence of increasing concentration of AMG 337, proved that binding of AMG 337 to the AT transporter inhibited AT function in human and canine cells.
Secondly, in vivo studies measured significant changes in blood pressure (which decreased after exposure to AMG 337) and heart rate (increased after exposure to AMG 337) in dogs. The magnitude of these responses was diminished by pre-treatment with theophylline, an AR antagonist.
Thirdly, ex vivo studies (conducted at REPROCELL Biopta’s Centre for predictive Drug Discovery in Glasgow, UK , see graphs below) on freshly dissected human subcutaneous arteries showed that AMG 337 caused a significant vasorelaxation induced by increasing concentration of AMG 337 in pre-constricted artery segments.
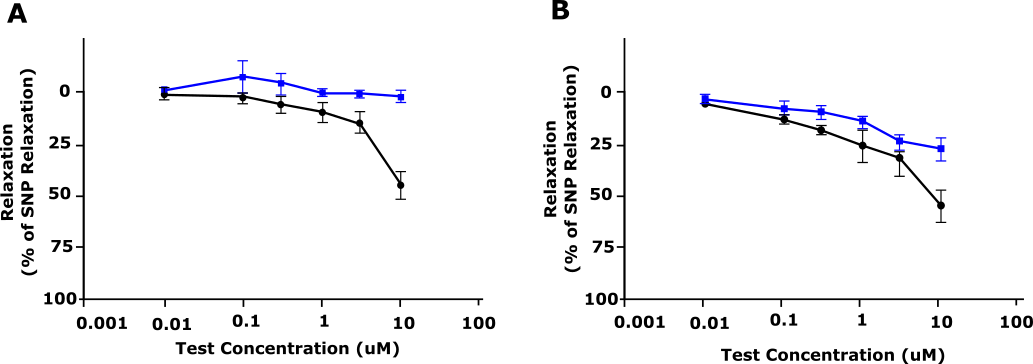
Figure 1: Relaxation response of AMG 337 (shown as % relaxation) in precontracted dog (A) and human (B) blood vessels. Blue, Vehicle; Black, AMG 33. Adapted from Amouzadeh et al, 2019.
Study Conclusions and Outcomes
Based on these non-clinical findings, the clinical investigators were advised to manage the headaches by administering caffeine, an AR antagonist (simply in the form of coffee!) to allow continuation of treatment in the first in human study. It was indeed reported that the use of caffeine alleviated the symptoms and permitted the continuation of the clinical trial.
This paper highlights the importance of ex vivo systems in order to accelerate (and reduce the cost of) drug discovery. The use of fresh human tissue specimens facilitates the assessment of efficacy and toxicity of novel treatment modalities in the context of conserved tissue architecture, cell signaling and extra-cellular matrixes, therefore making these screening systems clinically relevant and highly predictive.
Want to find out more?
To speak with one of our scientists about the benefits of human fresh healthy and diseased tissue in your translational research projects, please contact one of our experts. Alternatively, you can browse the resources below for more information.
References
- Kola et al. Can the pharmaceutical industry reduce attrition rates? Rev. Drug Discov. 3, 711–716 (2004).
- Amouzadeh et al. Clinical Implications and Translation of an Off-Target Pharmacology Profiling Hit: Adenosine Uptake Inhibition In Vitro. Oncol. 12, 1296–1304 (2019).
- Gherardi et al. Targeting MET in cancer: Rationale and progress. Nature Reviews Cancer 12, 89–103 (2012).
- Bean et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Natl. Acad. Sci. 104, 20932–20937 (2007).
- Smyth et al. Emerging molecular targets in oncology: Clinical potential of MEeT/hepatocyte growth-factor inhibitors. Targets. Ther. 7, 1001–1014 (2014).