Isolated blood vessels have been used for over a century to measure vascular contractility ex vivo, and rat aorta is commonly used due to its affordability and scalability.1,2 However, species differences can impact the clinical translatability of animal tissues. In this article, we will explore how isolated blood vessels can be used to determine drug mechanisms ex vivo and how results can vary based on the type of tissue used.
Why use studies in isolated arteries?
Isolated arteries are used by pharmacologists to determine the cardiovascular (CV) effects of potential new drugs, such as blood pressure changes, or to research basic mechanisms involved in the regulation of vascular tone. With respect to drug discovery, they can be used to detect off-target effects in CV safety studies or to predict therapeutic efficacy in cardiovascular diseases (CVD). Several different types of tissues are used in these organ bath studies, including rat aorta, rat mesenteric arteries, canine resistance arteries, and human resistance arteries.
What are CV Safety Studies?
CV safety studies are used as part of the preclinical drug discovery process to predict whether a drug has off-target CV effects before it is tested in humans. CV safety is one part of the "core battery" of safety pharmacology assessments required before an investigational new drug moves into clinical trials. Alongside central nervous system (CNS) and respiratory side effects, CV effects that may present an acute effect on the heart or vascular system (e.g. causing arrhythmia, a change in heart rate, or a change in blood pressure) are vital safety considerations.
FAQ: What is the role of safety pharmacology?
Safety pharmacology focuses on the acute effects that present an immediate risk to patients or volunteers during clinical trials. Longer-term toxicological effects are considered as part of the safety-tox process; even if toxic effects don't present an immediate risk to a patient or volunteer it is still important that the potential for such adverse effects is evaluated.
For example, any unintended agonism of the 5-HT2B receptor can prevent a drug from progressing clinically due to the risk that heart valves become adversely affected over the medium to long term.3 In vitro studies in fresh tissues tend to focus more on the acute safety effects of drugs, given the short-term nature of the experiments, which typically last hours. If a test article (i.e. a potential new drug therapy) does display off-target CV effects it may still proceed to clinical trials; however, the criteria for clinical advancement depends on a risk-benefit analysis.
What is Cardiovascular Disease (CVD)?
Cardiovascular disease is the number one cause of morbidity and was responsible for 30% of all known deaths in 2005 alone.4 Cardiovascular disease encompasses a range of fatal conditions, including coronary artery disease (CAD) and peripheral artery disease (PAD).5
Lifestyle changes and medication are commonly used to manage disease progression; however, many patients will eventually require surgical intervention such as arterial bypass surgery. This major procedure involves grafting another vessel, such as the saphenous vein, in place of the blocked artery.4 Like most conditions, non-surgical approaches to the treatment of this disease are preferred, but drug therapy and changes in lifestyle may not be sufficient to prevent disease progression.
Vasoconstriction in human saphenous vein
The model assesses whether test articles cause vasoconstriction in human saphenous vein, with sumatriptan (5-HT1B/1D receptor agonist) as a reference compound.
CV studies in isolated rat aorta
Of the various artery types, rat aorta is one of the most commonly used, with around 200 publications per annum (source: Pubmed). The isolated rat aorta model is a well-established methodology that has been used for over 100 years.1 It is popular due to the accessibility of rat tissue and it is relatively easy to work with in comparison to blood vessels from smaller animals such as mice.2
Methodology for rat aorta model
To begin this experiment, the abdominal and/or thoracic aorta is dissected from the surrounding tissues. If removing the endothelium, a cocktail stick can be gently rubbed around the vessel lumen to denude the aorta prior to suspension.5 Once isolated, the aorta is cut into ~4 mm rings which are attached between an organ bath and force transducer (figure 1).6 The transducer measures any changes in vascular tone, which indicate blood vessel constriction or dilation.
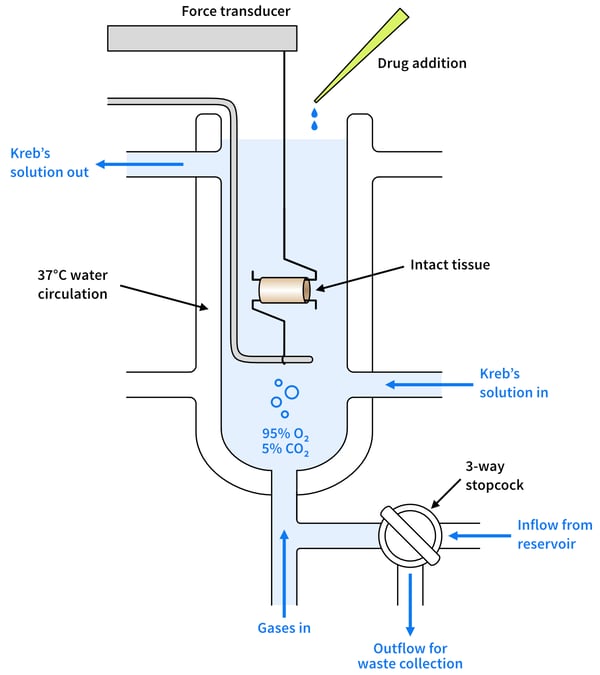
Figure 1: Cross-sectional diagram of a typical organ bath set-up
FAQ: What is an organ bath?
An organ bath is a heated chamber that mimics in vivo conditions. The chamber usually contains physiological saline solution (PSS) that is heated to 37°C and aerated with O2 /CO2 (95%/5% respectively).1 Test articles (i.e. drugs to be tested) and standard-of-care compounds are added to the PSS, exposed to the tissue for a set period to check the tissue viability, and are typically then washed out (if the test drug binds reversibly to its receptors) before the experimental protocol.
Most isolated strips or rings of tissues (minimum of 1-2 mm in diameter for a ring preparation) can be used in an organ bath for pharmacological studies. For tissues with a smaller diameter (of less than 1-2 mm), a wire myograph system is recommended.
The role of species differences in measuring vascular contractility ex vivo
Species differences refer to the genetic, pharmacological, and physiological variations between species that result in different drug responses. They can exist between any two species, but the term is most commonly used to refer to the differences between human and animal models.
When species differences go undetected, they can have significant consequences at clinical trial. For example, a drug may display no off-target effects in animal studies, but when it is tested in humans it causes side effects due to the activation of different receptors and/or pathways.9 It is, therefore, important to consider the mechanism of action (e.g., nitric oxide pathway) alongside the tissue response (e.g., vessel relaxation); especially during the later stages of drug development. The problem is that many preclinical researchers report on a drug's response without fully investigating the mechanism of action behind it.6-8
Rat aorta vs human arteries
On the surface, rat arteries behave similarly to human arteries ex vivo. Studies have shown that rat tissues produce the same changes in contractility as humans to common vasoconstrictors and vasodilators and even to many other less commonly used drugs such as cannabidiol (CBD),6 mevalonate,7 and FK506.8
When Baranowska-Kuczko et al. looked at the response of rat and human arteries to CBD, they discovered however that different pharmacological mechanisms were behind the relaxation responses observed.2 In humans, the response was endothelial-dependent and mediated by KCa and IP, EP4, and TRPV1 receptors.2 But in rats, relaxation was endothelium-independent and mediated by the CB1 receptor, suggesting a different mechanism of action between rats and humans.2
Species differences: canine vs human arteries
The below figure shows an example of pharmacological data generated in human tissue versus canine tissue. In this graph, we can see that the canine tissue (figure 2A, black line) shows a reduced relaxation response compared to the human tissue (figure 2B, black line) at the same concentration. In this example, the failure to identify these species' differences may in part explain why some participants in clinical studies experienced migraines whereas no effect was observed in preclinical animal studies. You can read more about this case study here.
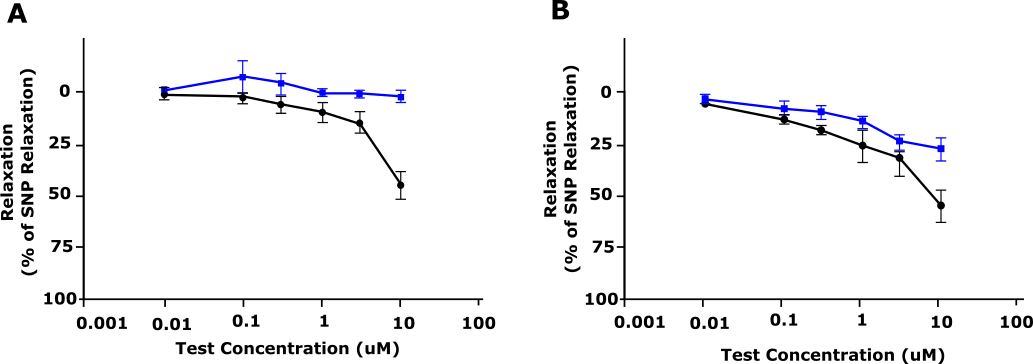
Figure 2: Relaxation response of AMG 337 (shown as % relaxation) in pre-contracted dog (A) and human (B) blood vessels. Blue, Vehicle; Black, AM G337. Adapted from Amouzadeh et al, 2019.9
Outsourcing research in human blood vessels
Although less easily accessed than rat aorta, studies using human blood vessels are more likely to yield translational results compared with studies using animal tissues. Examples of human vessels that can be used ex vivo include:
-
Subcutaneous resistance arteries
-
Coronary arteries
-
Saphenous veins
-
Pulmonary arteries
-
Chorionic plate arteries
If you are struggling to source donor arteries for human tissue research, this work can be outsourced to a contract research organization (CRO) like REPROCELL. To find out more about our human tissue services, visit our human tissue assay microsite, or contact one of our experts to arrange a free consultation.
Conclusion
The isolated rat aorta experiment is a well-established model for estimating the effect of compounds on vascular tone and has been the bedrock of cardiovascular tissue research for decades. This methodology can be used to test a range of tissue types ex vivo. Animal tissue can, however, lack clinical translation, meaning that drug behavior may be different when the same compound is tested in humans. As the trend towards the 3Rs continues, researchers can reduce the number of animal tissues used and improve the clinical translatability of their CV research by using human-isolated arteries.
References
- Dillmann WH. The rat as a model for cardiovascular disease. Drug Discovery Today: Disease Models 5:3 pp 1740-6757 (2009).
- Marta Baranowska-Kuczko et al. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: modification by hypertension and the potential pharmacological opportunities. Journal of Hypertension. 38 pp896–911 (2020).
- Cavero et al. Safety Pharmacology assessment of drugs with biased 5-HT2B receptor agonism mediating cardiac valvulopathy. Journal of Pharmacological and Toxicological Methods 69 pp 150-161 (2014).
- Katsimpoulas M et al. Investigation of the Biomedical Integrity of Decellularized Rat Abdominal Aorta Transplantation Proceedings 47:4 pp 1228-1233 (2015).
- National Health Service (NHS). Cardiovascular disease. NHS Online (2022).
- O'Sullivan et al. Time-dependant vascular actions of cannabidiol in the rat aorta. European Journal of Pharmacology 612 pp 61-68 (2009).
- Roullet et al. Mevalonate availability affects human and rat resistance vessel function. Journal of Clinical Investigation 96 pp 239-244 (1995).
- Lima JJ et al. Effects of FK506 in rat and human resistance arteries. Kidney International 55:4 pp 1518-27 (1999).
- Amouzadeh et al. Clinical Implications and Translation of an Off-Target Pharmacology Profiling Hit: Adenosine Uptake Inhibition In Vitro. Oncol 12 pp 1296–1304 (2019).




.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



