More than 200 million people are affected by peripheral artery diseases (PAD) worldwide [1]. This condition, caused by atherosclerosis of blood vessels in the lower half of the body, can result in coronary heart disease and cardiac morbidity [2,3]. Surgical intervention via stenting and balloon angioplasty is often recommended to avoid amputation [4]. However, artificial stenting carries significant post-operative complications, such as restenosis and the need for further re-vascularization procedures [5]. Alternative treatments are therefore required to negate these complications.
Researchers at Alucent Biomedical have developed a light-activated compound which could treat PAD without the need for artificial implants [6]. This small molecule strengthens blood vessels by linking natural fibers found in the extra-cellular matrix (ECM) of their lining. Upon exposure to a specific wavelength of light, this small molecule causes cellular proteins, such as collagen and elastin, to link, resulting in the formation of a natural vascular scaffold (NVS) [7]. Treatment with this compound (a substituted 1,8-naphthalimide) not only provides physical support to the affected blood vessel, but may even reduce inflammation in the affected area [6,7].
Results from a recent study suggest that NVS is safe for use in human blood vessels and does not affect normal cellular function [6,7]. The treatment is now being trailed clinically where it will be tested in living human subjects. If successful, it is hoped that NVS could be a safer alternative to stenting for patients with PAD.
In this article, we interviewed lead-author of the most recent study, Ejaz Ansari, to find out more about this fascinating novel treatment.

How did you determine that NVS was safe for use in human blood vessels?
Because NVS changes the blood vessel structure, our main concern was that it would affect blood pressure – which could produce dangerous side effects in humans.
Previously, the drug has been tested in porcine blood vessels[7] where it was shown to have no effect on natural blood vessel constriction – but we wanted to be sure. So in this study, we measured constriction in fresh, functional popliteal arteries from human donors.
It is important to note that there are ethical considerations when using tissues from human donors. We had to ensure that all tissues were donated with informed-consent under guidelines provided by the Human Tissue Authority and that patient data was completely anonymized.
What experimental methods did you use?
Our scientists used a tissue bath methodology in this experiment. Popliteal arteries from three coronary vascular disease ( CVD) donors were suspended in a tissue bath, pre-treated with NVS and light and exposed to a vasoconstrictor (U46619) and a vasorelaxant (sodium nitroprusside). This allowed us to test whether the drug had a significant effect on normal physiological activity – it did not (Figure 1).
The tissue bath method is an established, but highly specialized pharmacological method for estimating drug effects on blood pressure. We therefore had to ensure that all experiments had adequate controls in place and that tissue viability was confirmed.
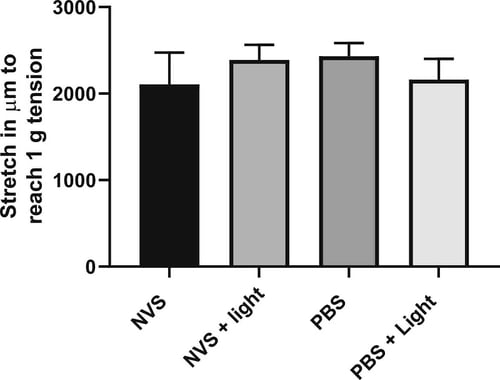 Figure 1: Stretch required (in μm) to reach 1 g (± 0.1 g) tension in isolated human popliteal artery rings pre-treated with NVS, NVS + light, PBS, or PBS + light. All groups showed similar stretch properties.
Figure 1: Stretch required (in μm) to reach 1 g (± 0.1 g) tension in isolated human popliteal artery rings pre-treated with NVS, NVS + light, PBS, or PBS + light. All groups showed similar stretch properties.
What quality control methods were put in place for this work?
Researchers at Alucent Biomedical used histological analysis to confirm the presence of atherosclerotic plaques, changes in extracellular matrix structure, and drug distribution within the donor tissue following treatment. Through this analysis, they confirmed that drug distribution was even, and that treated blood vessels had denser arterial walls than those exposed to the negative control.
One of the experimental outcomes showed that the drug may reduce inflammation – how did you measure this?
Following treatment, we cultured some artery rings in cell culture media and measured the levels of interleukin-6 (IL-6) in the supernatants to check for any drug induced inflammation. ELISA results showed reduced cytokine levels in NVS treated samples suggesting NVS may exert an anti-inflammatory effect.
As a result of this study’s success, Alucent Biomedical are now trialing NSV in human subjects. Their phase one trial will test NVS in 15 patients with symptomatic PAD, using a single group assignment method. It’s a very exciting time for Alucent Biomedical – I wish them every luck with this clinical trial and any future work.
%20pre-treated%20with%20NVS%2c%20NVS%20light%2c%20PBS%2c%20or%20PBS%20light.jpg?width=500&height=379&name=IL-6%20levels%20in%20supernatants%20collected%20from%20cultured%20human%20isolated%20popliteal%20artery%20rings%20(n%20=%203)%20pre-treated%20with%20NVS%2c%20NVS%20light%2c%20PBS%2c%20or%20PBS%20light.jpg) Figure 2: IL-6 levels in supernatants collected from cultured human isolated popliteal artery rings (n = 3) pre-treated with NVS, NVS + light, PBS, or PBS + light. Blood vessels treated using NVS displayed a non-significant reduction in inflammation.
Figure 2: IL-6 levels in supernatants collected from cultured human isolated popliteal artery rings (n = 3) pre-treated with NVS, NVS + light, PBS, or PBS + light. Blood vessels treated using NVS displayed a non-significant reduction in inflammation.
References
- Fowkes et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382:1329–1340 (2013).
- National Health Service. Peripheral arterial disease (PAD): Overview (2019). Available at: https://www.nhs.uk/conditions/peripheral-arterial-disease-pad/
- Regensteiner et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vascular Medicine 13:15–24. (2008).
- Goodney at al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Cardiovascular Quality and Outcomes 5:94–102 (2012).
- Gerhard-Herman et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135 (2017).
- Ejaz et al. Retained Functionality of Atherosclerotic Human Arteries Following Photoactivated Linking of the Extracellular Matrix by Natural Vascular Scaffolding Treatment. Journal of Cardiovascular Translational Research (2020).
- Munger et al. A novel photochemical cross-linking technology to improve luminal gain, vessel compliance, and buckling post-angioplasty porcine arteries. Journal of Biomedial Materials Research Part B Applied Biomaterials 104:B375–B384 (2016).



.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



