iPSC Purified Exosomes (Lyophilized)
RCEVLY001
Brand: REPROCELL®
Human iPSC-derived lyophilized exosomes for preclinical and advanced research applications.

| Product name | Catalog number | Pack size | Price | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|---|
| iPSC Purified Exosomes (Lyophilized) | RCEVLY001 | 10 mL vial | Ask us | $ Ask us | £ Ask us | € Ask us |
Note: prices shown do not include shipping and handling charges.
REPROCELL’s iPSC-Derived Exosomes: GMP-compliant Exosomes Derived from StemRNA Clinical iPSC Clones.
Our iPSC-derived exosomes are highly purified and concentrated from iPSCs, ensuring quality, reproducibility, consistency, and safety for preclinical and advanced research applications. Ideal for studying anti-aging and collagen production. They are manufactured in a licensed facility under rigorous quality control.
Manufactured using clinical grade iPSC in compliance with FDA, EMA, and Japanese PMDA
REPROCELL's iPSC-derived exosomes are produced using our StemRNA Clinical iPSC Clones that fully comply with the regulations of Japan, the United States, and Europe. Manufacturing of clinical-grade iPSCs begins with comprehensive donor screening and testing to ensure the absence of viruses, bacteria, and potential human pathogens. Given that regulatory authorities in different regions maintain distinct lists of designated infectious diseases, we perform multiple rounds of testing to align with FDA, EMA, PMDA, and other international requirements. This rigorous approach safeguards product integrity and ensures global regulatory compliance.
Only iPSCs that successfully meet these stringent testing criteria progress into REPROCELL’s exosome manufacturing process, ensuring the highest standards of safety, consistency, and quality for both research and clinical applications.
The manufacturing of our iPSC-derived exosomes is performed in a dedicated REPROCELL Japan GMP Manufacturing Facility licensed under the Act on the Safety of Regenerative Medicine by the Ministry of Health, Labour and Welfare (MHLW). This license authorizes the facility to manufacture specified processed cell products and to provide contract cell manufacturing for both clinical-research applications and private medical treatment. All production activities are conducted under a rigorous quality and safety management system.
This product is also available as a suspension.
Features of REPROCELL's iPSC exosomes
- Anti-ageing potential
- High purity formulation
- Rigorous quality control for consistency and safety
- Manufactured in a licensed facility (MHLW Specified Cell Product Manufacturing License No. FA3200006” (MHLW-SCPL No. FA3200006)
-
Produced from REPROCELL's StemRNA Clinical iPSC Clones, generated via our clinical StemRNA™ Reprogramming Technology
For more details visit: REPROCELL Japan GMP Manufacturing Facility.
High Purity and Concentration
Once the iPSCs are cultured, the cells are removed by centrifugation, and the culture supernatant is collected. This supernatant contains a range of bioactive substances secreted by the cells — including cytokines, growth factors, proteins, metabolites, and extracellular vesicles (exosomes) (Figure 1).
Exosomes represent only a small fraction of the total components. The culture supernatant is a complex mixture, and exosome isolation requires additional purification and concentration steps to separate them from other extracellular vesicles and soluble factors.
REPROCELL provides highly purified, concentrated exosomes from iPSCs, ensuring quality, reproducibility, consistency, and safety for preclinical and advanced research applications.
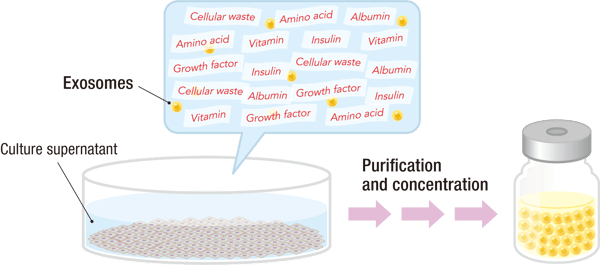
Figure 1. Exosomes are isolated and purified from the iPSC culture supernatant to provide a highly purified, concentrated exosomes.
Analysis of REPROCELL’s purified iPSC-derived exosomes confirmed that levels of several components originally present in the culture medium dropped below the detection limit after purification (Figure 2A). Additionally, particle size analysis shows that the product contains a high proportion of vesicles in the 50–150 nm range, consistent with the typical size of exosomes (Figure 2B).
A |
B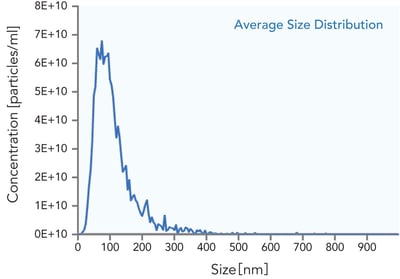 |
Figure 2. Purification Efficiency and Size Distribution of iPSC-Derived Exosomes: Residual Media Components Undetectable and NTA-Confirmed Particle Size of 50–150 nm. A. Left Panel: Residual media components after purification of REPROCELL iPSC-derived exosomes. Most additives fall below detection limits, confirming effective purification. B. Right Panel: Nanoparticle size analysis of purified exosomes. The majority of particles fall within the expected exosome size range of 50–150 nm.
Proteome Analysis Of Exosomal Cargo
Exosomes encapsulate a rich cargo of biologically active molecules (Figure 3A). Analysis of REPROCELL's iPSC exosomes revealed a diverse range of functional factors, including those that promote anti-inflammatory responses, inhibit apoptosis (cell death), and support DNA repair. iPSC-derived exosomes contain a much wider variety of proteins than MSC-derived exosomes (another commonly used source for exosomes) [REF 1, 2, 3] (Figure 3B). The analysis results of major proteins detected in the proteome analysis are shown (Figure 3C).
A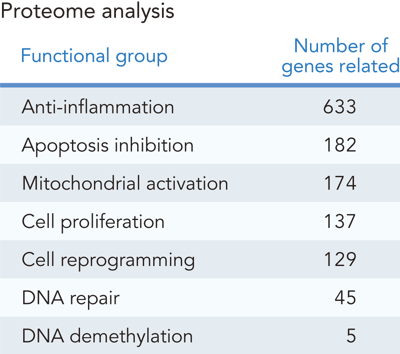 |
B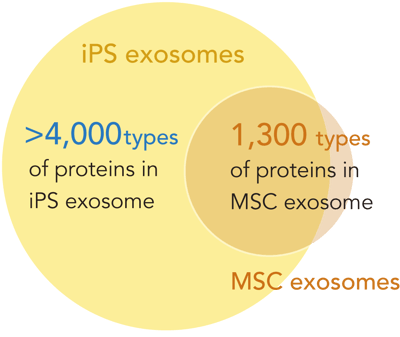 |
Figure 3. Proteome analysis of REPROCELL's iPSC exosomes.
Relevant Pages
- REPROCELL Japan GMP Manufacturing Facility
- Understanding iPSC-derived Exosomes
- FAQ: iPSC Exosomes - Preclinical Research Overview
Analysis results of major proteins detected in the proteome analysis of our iPS exosome products:
| Functional Category | Major Genes | Function |
| Pluripotency and Cell Plasticity Factors | LIN28A, POU5F1, SALL4, etc. | A group of factors known to regulate pluripotency and self-renewal capacity. Key characteristics of iPS cells. |
| Cellular and Energy Metabolism Regulators | NAMPT, G6PD, PKM, etc. | A group of enzymes involved in regulating intracellular metabolism. Includes NAMPT (involved in NAD synthesis) and G6PD (pentose phosphate pathway), related to cellular energy metabolism (ATP production) and biosynthetic pathways. |
| Genome Stability and DNA Repair Factors | XRCC5/6, PARP1, MCM complex, etc. | A group of factors reported to be involved in DNA damage response and repair mechanisms. Related to maintaining genomic stability and cellular stress response. |
| Protein Quality Control (Molecular Chaperones) | HSP family, CCT complex, etc. | Factors known as "molecular chaperones" that assist in protein folding. Related to maintenance mechanisms of intracellular proteostasis and cellular stress tolerance. |
| Redox Control and Oxidative Stress Response Factors | SOD1/2, PRDX family, GSTP1, etc. | Rich in enzymes involved in controlling reactive oxygen species (ROS). Related to the metabolic pathways of superoxide and hydrogen peroxide. |
| Autophagy and Intracellular Transport Factors | RAB7A, ATG3, LAMP1, etc. | Factors related to autophagy and lysosomes (intracellular degradation and recycling systems). Involved in intracellular quality control mechanisms and substance transport. |
| Immunomodulation and Cell Adhesion Molecules | ANXA1, MFGE8, ICAM1, etc. | Includes Annexin A1 (ANXA1), involved in regulating immune responses, and adhesion molecules that mediate cell-cell interactions. Related to signal transduction in the cellular microenvironment (niche) and tissue engineering. |
| Cell Proliferation and Translation Control Signaling Factors | mTOR, 14-3-3 proteins, EIF5A, etc. | Signal transduction factors involved in the regulation of cell growth and protein synthesis. Related to cell proliferation mechanisms and intracellular signaling networks responding to nutritional status. |
| Proteasome Components | PSMA1, etc. | Includes factors that constitute the ubiquitin-proteasome system (UPS) and are involved in protein degradation. Related to intracellular protein turnover and the disposal of unwanted proteins. |
References
- Kim, S., Lee, S.K., Kim, H. and Kim, T.M. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. International Journal of Molecular Sciences. 19, 2018.
- Liu, S., Mahairaki, V., Bai, H., Ding, Z., Li, J., Witwer, K.W. and Cheng, L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells. 37, 779-790. 2019.
- Wang, S., Hou, Y., Li, X., Song, Z., Sun, B., Li, X. and Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging (Albany NY). 12, 19546-19562. 2020.
REPROCELL company name and logo is the property of REPROCELL Inc., Japan.
Product Name: iPSC Purified Exosome (Lyophilized)
Product Number: RCEVLY001
Quantity: 10 ml vial
Formulation: Lyophilized powder from saline suspension
Particle Size: 50–150 nm (extracellular vesicles)
Source: StemRNA™ Clinical iPSCs
Composition: Proteins, nucleic acids, and bioactive molecules
Key Functions: Anti-inflammatory, anti-apoptotic, supports DNA repair
Manufacturing Standard: GMP-compliant facility
Quality Control:
- Sterility testing: Sterile
- Endotoxin testing: < 0.01401 EU/mL
- Mycoplasma testing: Negative
- Pathogen testing (HIV 1,2; HTLV1,2; HBV; HCV; EBV; CMV; Parvo B19): Negative
Intended Use: Research and preclinical studies in regenerative medicine