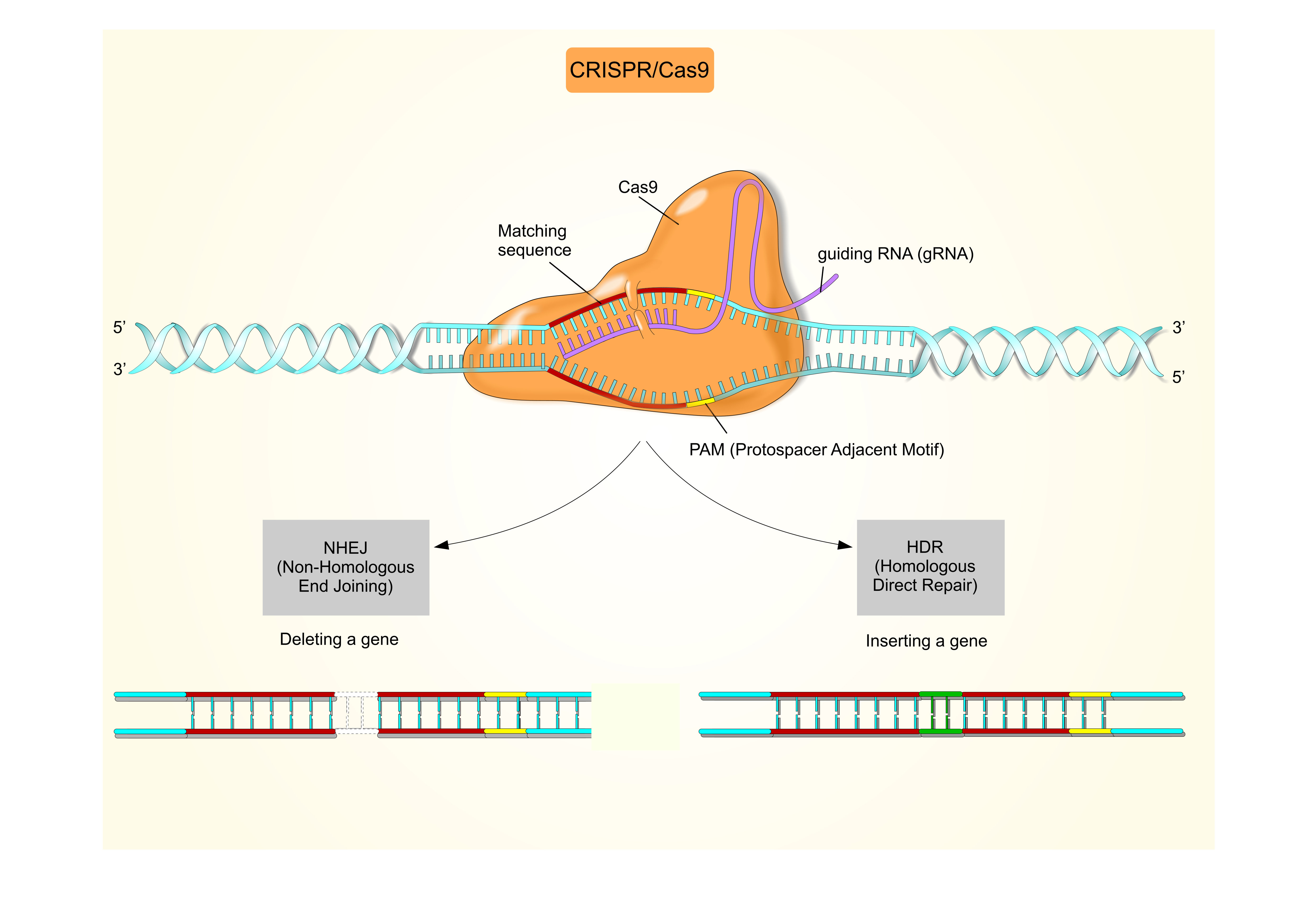
Thanks to the discovery of CRISPR-Cas9, gene editing is more accessible than ever before. However, some genetic modifications remain challenging and there are even more factors to consider if your cells are intended for clinical use. By outsourcing your clinical gene editing to REPROCELL, you can achieve the genotype you need before moving on to expensive cell bank manufacturing processes.
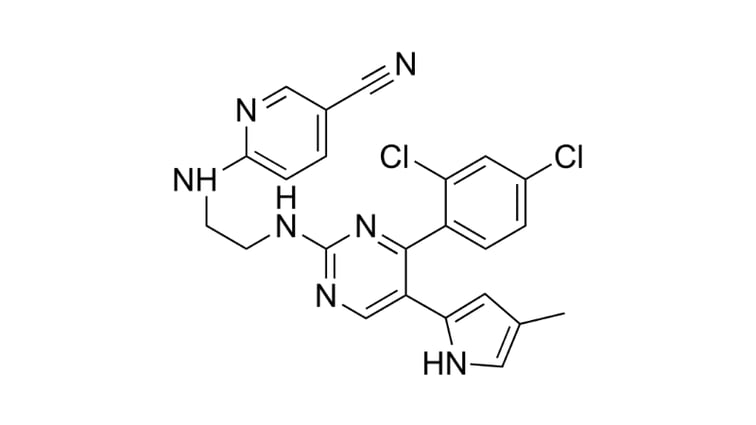
Our StemEdit clinical gene editing service uses advanced CRISPR-SNIPER* gene editing technology to develop your engineered stem cells. Due to the increased screening specificity of CRISPR-SNIPER, we can successfully achieve complex genetic edits with high accuracy at an early stage. By evaluating the percentage of target cells and determining their transfection efficiency (go/no-go decision point) StemEdit saves time as the SNIPER pre-screen is performed before laborious clone selection.
*SNIPER = Specification of Newly Integrated Position and Exclusion of Random-integration. Note: CRISPR-SNIPER modified cells are developed, manufactured or supplied by GenAhead Bio Inc. under license from ERS Genomics Limited and Broad Institute.
REPROCELL Stemgent™ – the stem cell experts
Our broad range of products and services for stem cell scientists are used by leading pharmaceutical and biotechnology companies as well as top academic and government research institutions all around the globe.
Case study:
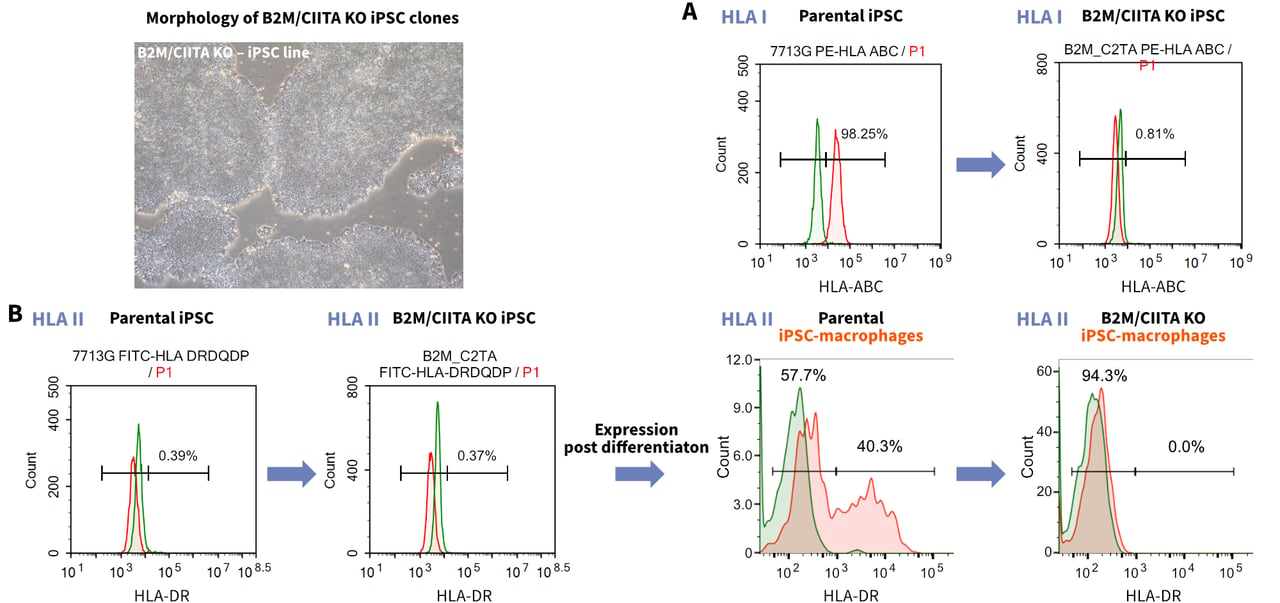
HLA Disruption in clinical Seed Stock iPSCs
One limitation of using iPSCs clinically is immunogenicity caused by Human Leucocyte Antigen (HLA) mismatching, which can reduce the in vivo survival and therapeutic efficacy of these cells.1,2 The immunogenicity of iPSCs can be reduced by disrupting the genes responsible for immune recognition using CRISPR-Cas9. One approach is to knock out the B2M (β2 microglobulin) and CIITA (major histocompatibility complex [MHC] II transactivator) genes.
In humans, the B2M protein is required for the presentation of HLA protein A-G on the cell surface, while CIITA is essential for HLA-II transcription.2,3 Dual knock-out of the B2M and CIITA genes disrupts the presentation of MHC-I/MHC-II proteins in iPSCs, improving their therapeutic potential while maintaining pluripotency.1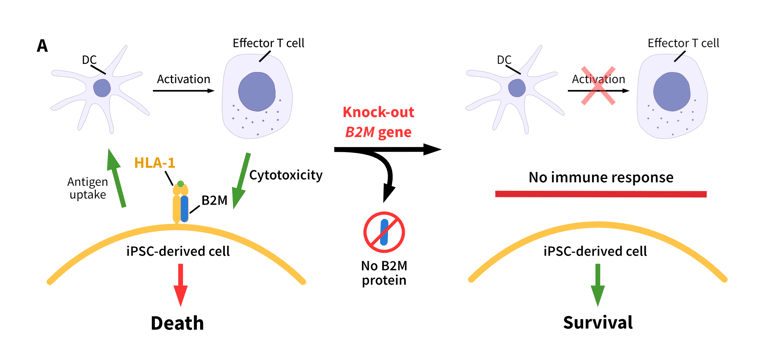
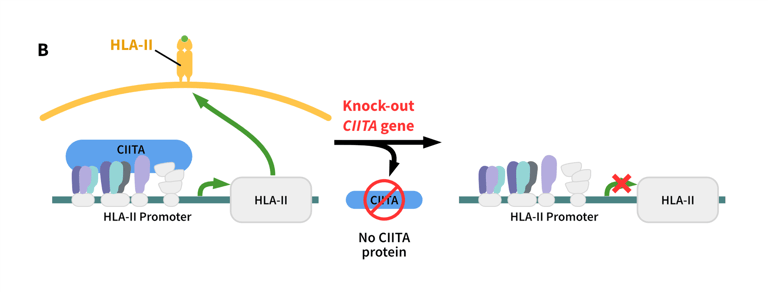
Role of B2M and CIITA in the regulation of the immune response. B2M regulates the activation of effector T cells. B2M knockouts do not activate effector T cells, leading to a reduced immune response. CIITA regulates the expression of the HLA-II complex. Knockout of CIITA leads to a reduced expression of HLA-II, and a reduced immune response.
References
- Wang X et al. Diminished expression of major histocompatibility complex facilitates the use of human induced pluripotent stem cells in monkey. Stem Cell Research & Therapy 11:334 (2020).
- Xu H et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 24 pp566-578 (2019).
- Deuse et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nature Biotechnology 37 pp 252–258 (2019).