Bioengineering of human full thickness skin for in vitro applications, screening and product development
● Download this protocol as a PDF (0.25 MB)
1. Materials
| Alvetex Scaffold 12 well insert (note: 12 well inserts can be suspended in wells of a 6 wellplate) |
REPROCELL AVP005 |
| 6 well plate | e.g. Greiner BioOne 657160 |
| Alvetex well insert holder and deep Petri dish | REPROCELL AVP015-2 |
| Human Fibroblast Expansion Basal Medium | Life Technologies M-106-500 |
| Low Serum Growth Supplement | Life Technologies S-003-10 |
| EpiLife Medium | Life Technologies M-EPI-500 |
| Human Keratinocyte Growth Supplement | Life Technologies S-001-5 |
| Gentamicin/ Amphotericin Solution | Life Technologies R-015-10 |
| KGF (diluted to 10 μg/mL in 1× DPBS) | Life Technologies PHG0094 |
| CaCl2 Solution | 2 M Stock |
| TGFβ1 Recombinant Human Protein | Life Technologies PHG9214 |
| Ascorbic Acid (Vitamin C) | Sigma A4544 |
| Trypsin EDTA | Life Technologies R-001-100 |
| Trypsin Neutraliser | Life Technologies R-002-100 |
| Human Dermal Fibroblasts (HDFn, HDFa) | Life Technologies neonatal: C-004-5C adult: C-013-5C |
| Human Keratinocytes (HEKn) | Life Technologies neonatal: C-001-5C |
2. Schematic
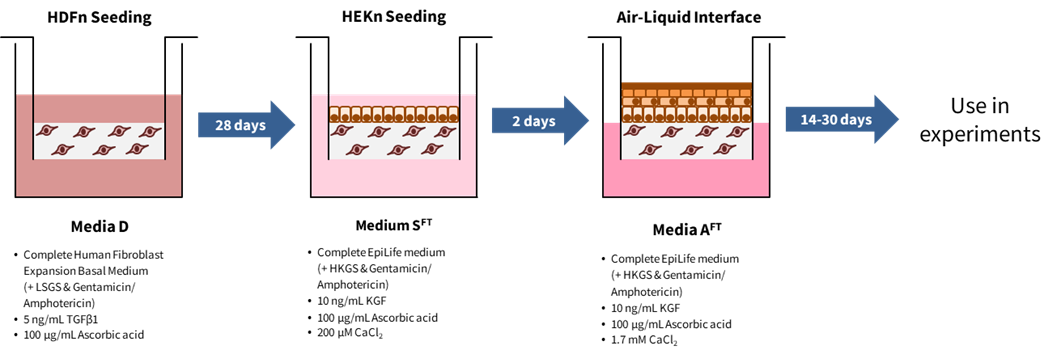
3. Protocol
A. Preparation of Alvetex Scaffold
Alvetex Scaffold requires wetting pre-treatment as follows:
- Soak in 70 % ethanol for 2-5 minutes in a deep Petri dish.
- Soak in 1 × sterile PBS for 2-5 minutes in a deep Petri dish.
- Place Alvetex Scaffold well inserts in a 6 well plate and pass 1 mL Human Fibroblast Expansion Basal Medium through each Alvetex Scaffold disc.
- When ready to seed cells, aspirate excess media from the wells.
B. Seeding of fibroblasts onto Alvetex Scaffold
- Prepare 11 mL media D per Alvetex Scaffold well insert.
Media D:
Complete Human Fibroblast Expansion Basal Medium (+ LSGS & Gentamicin/Amphotericin)
5 ng/mL TGFβ1
100 µg/mL Ascorbic acid
| Stock solution concentration | Final concentration | Dilution | |
| TGFβ1 | 10 μg/mL | 5 ng/mL | 2000 × |
| Ascorbic acid | 10 g/mL | 100 μg/mL | 100 × |
- Harvesting fresh HDFn cell cultures:
- Aspirate growth medium from the cultures.
- Wash once with 3 mL trypsin-EDTA (10 %, 0.25 % trypsin; 90 % versene) and aspirate off.
- Treat with 5 mL trypsin-EDTA (for a T175 flask) until detached (approx. 8-10 minutes).
- Add 5 mL complete media.
- Transfer cell suspension to a centrifuge tube.
- Rinse flasks again with complete media.
- Spin down cells at 200 g for 5 minutes.
- Resuspend cell pellets in complete media and perform cell counts using a traditional hematocytometer and Trypan Blue exclusion assay (usually resuspend in 1 mL/T175 and 1:10 Trypan Blue dilution to ensure a reasonable density of cells for counting).
- 160 μL media
- 20 μL Trypan Blue
- 20 μL cell suspension
- Adjust cell suspension volume to 500,000 cells per 100 μL.
- Seed fibroblasts (100 μL) onto Alvetex Scaffold in a dropwise manner, ensuring even coverage.
- Place in 37°C incubator (5 % CO2, 95 % relative humidity) and allow cells to attach for minimum 2 hours.
- Carefully add media D to the outer compartment (i.e. outside and below the Alvetex Scaffold insert while suspended in a well of a 6 well plate). When media reaches the bottom of the scaffold gently add ~0.5 mL to the inner compartment (within the insert, where the cells are). Continue to fill outer compartment to the maximum level (i.e. so that the outer and inner medium becomes continuous through the “side windows” of the well insert).
Note: 10 mL media used per 6 well plate well/scaffold. Ensure no air bubbles form under the scaffold when you add the media.
- Place at 37°C (5 % CO2, 95 % relative humidity), and change media every 3-4 days (usually Monday and Friday).
- Culture for 28 days before seeding HEKn cells.
Note: Some fibroblasts may fall through the scaffold and begin to grow on the culture plate well floor. To avoid these cells affecting growth of the dermal equivalent, move scaffold to a fresh 6 well plate after 2 days.
Note: Over the 28-day culture period fibroblasts will start to grow in the culture well, it is recommended that models are transferred to a new well every 2 weeks.
C. Seeding of keratinocytes onto dermal component
- Prepare 11 mL media SFT per dermal equivalent.
Media SFT:
Complete EpiLife medium (+ HKGS & Gentamicin/ Amphotericin)
10 ng/mL KGF
100 µg/mL Ascorbic acid
200 μM CaCl2
Note: EpiLife medium contains 60 μM CaCl2; add 140 μM.
| Stock solution concentration | Final concentration | Dilution | |
| KGF | 10 μg/mL | 10 ng/mL | 1000 × |
| CaCl2 | 2 M | 140 μM | 14300 × |
| Ascorbic acid | 10 mg/mL | 100 μg/mL | 100 × |
- Harvesting fresh HEKn cell cultures (p3 in flask p4 in dermal equivalent model):
- Aspirate growth medium from the cultures.
- Wash once with 3 mL Trypsin-EDTA (10 %, 0.25 % trypsin, 90 % versine).
- Incubate at 37°C with 5 mL Trypsin-EDTA (for a T175 flask) until detached.
- Add 5 mL Trypsin neutraliser solution.
- Transfer cell suspension to a centrifuge tube.
- Further rinse flasks with complete EpiLife medium.
- Spin down cells at 200 g for 5 minutes.
- Resuspend cell pellets in media SFT and perform cell counts using a traditional hematocytometer.
- Aspirate media from both the surrounding well and the top of the dermal equivalent. Keep the dermal equivalent in the 6 well plate and add HEKn cells.
- HEKn cells are seeded at a density of 1.3 × 106 cells per 12 well insert (dermal equivalent model) in seeding volume 100-300 μL.
- Place in 37°C incubator (5 % CO2, 95 % relative humidity) and allow cells to attach for 2 hours.
- Complete by adding 10 mL of media SFT to each well containing an Alvetex Scaffold insert.
- Carefully check the underside of each Alvetex disc insert, without lifting it out of the medium, to ensure that no air bubbles are trapped below the insert.
- Incubate in submerged culture at 37°C, 5 % CO2 and 95 % relative humidity for 48 hours.
D. Going to the Air-Liquid Interface
- Prepare 36 mL media AFT per deep Petri dish (note: the Alvetex well insert holder can hold 3 inserts).
Media AFT:
Complete EpiLife medium (+ HKGS & Gentamicin/Amphotericin)
10 ng/mL KGF
100 µg/mL Ascorbic acid
1.7 mM CaCl2
Note: EpiLife medium contains 60μM CaCl2; add 1.64 mM.
| Stock solution concentration | Final concentration | Dilution | |
| KGF | 10 μg/mL | 10 ng/mL | 1000 × |
| CaCl2 | 2 M | 1.64 mM | 1219 × |
| Ascorbic acid | 10 mg/mL | 100 μg/mL | 100 × |
- Using a sterile pair of forceps transfer Alvetex Scaffold 12-well inserts to an Alvetex Well Insert Holder in a deep Petri dish on medium height. Note: three inserts can be cultured per deep dish.
 An Alvetex well insert holder and deep Petri dish allows for three height settings. The full thickness model is cultured using the medium setting.
An Alvetex well insert holder and deep Petri dish allows for three height settings. The full thickness model is cultured using the medium setting.
- Tilting the plate to the side aspirate the medium from inside each insert, taking care not to puncture the membrane.
- Add 35 mL media AFT per dish. Note: the media should only just touch the bottom of the model.
- Incubate at 37°C, 5% CO2 and change medium twice a week. Note: Monday and Friday media changes work well.