Alvetex Scaffold Application Note 09
Testing cytotoxicity in MCF-7 and SW620 cancer cell lines in Alvetex Scaffold 96 well plate format
Download this application note as a PDF (0.55 MB)
Certain aspects of cancer research rely on animal models but these have associated feasibility and ethical concerns [1,2]. Another widely used in vitro system is the clonogenic assay [3] which has been widely used to determine drug response of tumours. However, such issues as low frequency of evaluation, clump artefacts, lack of cytotoxic end points and lack of normal cell-cell interactions (which exist in a normal tissue environment), provide a need for the development of more realistic three-dimensional (3D) in vitro culture systems that can more accurately predict in vivo resistance and allow a controlled approach to investigating cancer cell biology, compound screening and disease modelling.
The vast majority of such models involve culturing cells on conventional two-dimensional (2D) plasticware in which cells adapt to the flat polystyrene substrate, flatten and grow as monolayers. Unfortunately, this approach is a poor surrogate and does not mimic the environment that cells experience in vivo. The morphology of cultured cells as monolayers is not realistic, cell-to-cell contact is limited and the microenvironment generated by the cells, for example via extracellular matrix (ECM) deposition, is reduced and altered [4].
In this application using Alvetex Scaffold 96-well plate technology, we demonstrate the 3D growth of the popular breast cancer cell line, MCF-7 and the colon cancer cell line SW620. We compare the viability of these cells with equivalent cultures grown on conventional 2D plasticware. The data show that MCF-7 and SW620 cells cultured on Alvetex Scaffold 96-well plate possess a different sensitivity pattern to certain cytotoxic drugs than their 2D counterparts that is more likely to resemble in vivo responses. This underlines the value of Alvetex Scaffold technology by providing a superior culture environment for cancer cell research and providing a tool to enhance in vitro sensitivity assays in cancer.
Method
MCF-7 cells (ATCC) were cultured at 50,000 cells/well for a period of 3 days prior to exposure to doxorubicin (1-10 μM) and tamoxifen (10-50 μm) for a period of 72 hours. SW620 cells were cultured at 50,000 cells/well for a period of 3 days prior to exposure to doxorubicin (1-10 μM) for 72 hours. Cell viability was assessed using the MTT assay (see Section 4.1 of the protocol, Alvetex Scaffold 96-well plate: Instructions for Use and Sample Applications) and the percentage viability calculated against a non drug treated control (designated 100 % viability). Tamoxifen, a commonly used drug in hormone receptor-positive breast cancer, is an antagonist of the estrogen receptor in breast tissue by the common metabolite hydroxytamoxifen. Doxorubicin, another commonly used drug in cancer chemotherapy is an anthracycline antibiotic and works by intercalating DNA.
MCF-7 cells show a different response to exposure to doxorubicin compared to tamoxifen. When exposed to doxorubicin, a more sensitive initial response was observed at 1 and 2 μM concentration with MCF-7 cells in Alvetex Scaffold, but with a greater percentage of viable cells at further increasing concentrations compared to MCF-7 cells cultured in 2D plates. 100 % cell death was not achieved in either MCF-7 cells grown in 2D or Alvetex Scaffold format at the concentrations tested. After 3 days tamoxifen exposure, MCF-7 cells grown on Alvetex Scaffold 96-well plate format show greater resistance at all tamoxifen concentrations. 100 % cell death was achieved at an approximate concentration of 23.9 μM with MCF-7 cells grown in 2D compared to Alvetex Scaffold 96-well plate format where 100 % cell death was not achieved at the maximum concentration of tamoxifen used (50 μM).
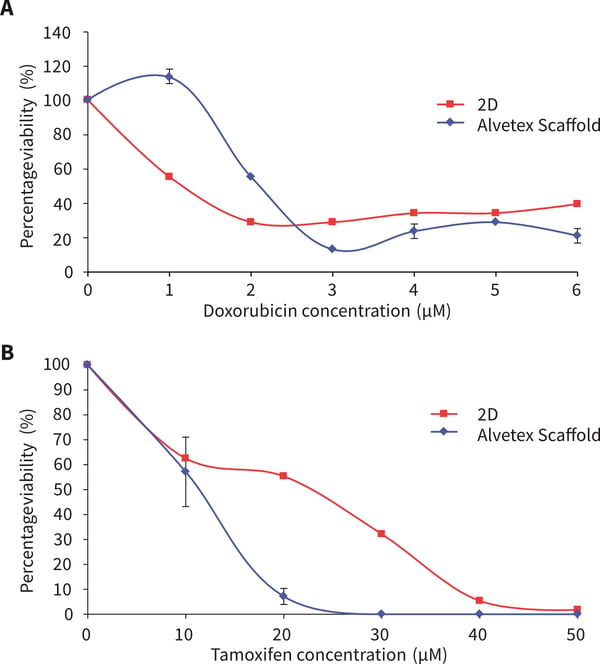
Figure 1. MCF-7 cells after 72 hours culture period and exposure to the following drugs for 72 hours (A) doxorubicin (B) tamoxifen. Data represents n=6, ± SE. IC50 values (A) 2.1 μm and 1.1 μM for 2D and Alvetex Scaffold, respectively, (B) 11.7 μM and 23.9 μM for 2D and Alvetex Scaffold, respectively.
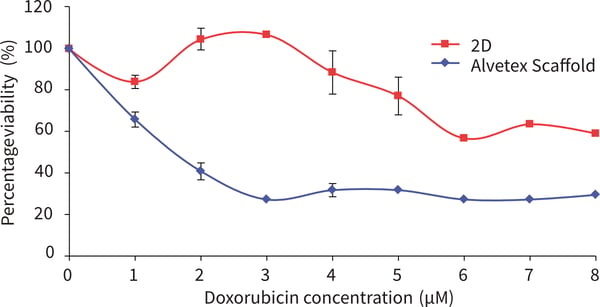
Figure 2. SW620 cells after 72 hours culture period and exposure to doxorubicin for 72 hours. Data represents n = 6, ± SE (IC50 values, 1.5 μM for 2D, for Alvetex Scaffold cell viability remains above 50 %). When exposed to doxorubicin SW620 cells show far greater resistance to all concentrations of doxorubicin tested compared to SW620 cells grown in 2D culture.
Conclusions
Overall, Alvetex Scaffold 96-well plate technology provides a unique platform which is compatible with standard in vitro assay methods used when culturing tumour cells to assess and screen a large range of test compounds. However, the sensitivity patterns obtained on Alvetex Scaffold 96-well plate technology are different to standard 2D assay responses, showing this three-dimensional technology could provide a more sensitive
and accurate tool for the use of in vitro sensitivity assays in cancer research and drug development.
References
- Strebhardt K, and Ullrich A. Paul Ehrlichʼs magic bullet concept: 100 years of progress. Nat Rev Cancer, 2008.
8(6): p. 473-80 - Lord CJ, and Ashworth A. Biology-driven cancer drug development: back to the future. BMC Biol, 2010. 8: p.38
- Hoffman RM. In vitro sensitivity assays in cancer: A review, analysis and prognosis. J Clin Lab Anal, 1991. 5: p. 133-143.
- Knight A. Systematic reviews of animal experiments demonstrate poor human clinical and toxicological utility. Altern Lab Anim, 2007. 35(6): p. 641-59.