In the following blog article, we have summarized the meaning and importance of peripheral blood mononuclear cells (PBMCs), plus the most commonly used blood products and methods for their isolation.
What does PBMC mean?
PBMC is short for "Peripheral Blood Mononuclear Cell", or any blood cell with a single nucleus.1 PBMCs are less dense than the other cells found in our peripheral circulation, such as red blood cells (RBCs) and granulocytes.2 Many of our immune cells are PBMCs including lymphocytes, monocytes, and dendritic cells.2
While PBMCs are typically isolated from the peripheral circulation, they can also be derived from buffy coat, bone marrow and cord blood samples.1 The distribution of cells within a PBMC isolate can differ depending on the source material. Table 1 shows the cellular subtypes contained within the PBMC fraction and their typical distribution in healthy adults.
|
Cell Subset |
Percentage (%) | Number of cells isolated from 1mL of blood (x103) |
| T Cells | 60 | 300-1200 |
| T Helper Cells | 70 of T cells | 210-840 |
| Cytotoxic T Cells | 30 of T cells | 90-360 |
| Monocytes/Macrophages | 15 | 75-300 |
| B Cells | 10 | 50-200 |
| Natural Killer Cells | 15 | 75-300 |
Table 1: Average composition of PBMCs in a healthy adult. Adapted from Bittersohl et al (2016)2
Why are PBMCs important in clinical research?
Over the past 50 years, PBMCs have become increasingly important in clinical research. A quick screen of PubMed records reveals thousands of studies exploring their use in a clinical research setting (figure 2).
One reason for their increasing popularity is that PBMCs are needed to develop the CAR-T cells used in cell and gene therapies. They are also a valuable resource in drug development as they provide a snapshot of someone's immune response. Other uses include, but are not limited to:
- Patient stratification
- Biomarker identification
- Immuno-oncology
- Toxicology
- Rare disease research
PBMCs must be of high quality, purity, and viability for use in a clinical research setting, however, they are fragile and can be impacted by several factors. Therefore, these cells should only be handled by suitably trained personnel in laboratories that comply with the principles of Good Clinical Laboratory Practices (GCLP).
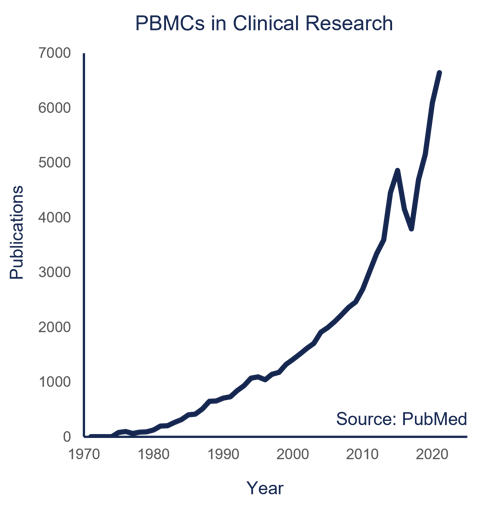 Figure 2: The number of publications made on PBMCs in Clinical Research has accelerated over the last 50 years. Source: PubMed.
Figure 2: The number of publications made on PBMCs in Clinical Research has accelerated over the last 50 years. Source: PubMed.
Main biospecimen sources of PBMCs
The most common starting material for PBMC isolation is peripheral blood, however, PBMCs can also be isolated from other blood products. No matter the source of PBMCs, there are different processing options. Below we have listed the four main sources for PBMC isolation and their typical concentration of PBMCs (figure 3).
- Whole blood
Whole blood samples are usually collected in specialized tubes at various volumes and contain an anticoagulant such as K2EDTA or LiHep. The number of PBMCs per milliliter of blood can vary depending on patient age (e.g., pediatric patients have more PBMCs compared to older patients) but typically PBMCs can be isolated from adult whole blood at a concentration of 0.5-3x106 cells/mL. - Buffy coat
The buffy coat is a side-product of blood donation and can be isolated from whole-blood samples. There is a much higher concentration of PBMCs in these samples compared with whole blood. Typically, a 50mL sample can include up to 500 million PBMCs, with RBCs composing <10% of the total sample volume. - Leukoreduction system chamber
Leukoreduction system chambers can be used to isolate PBMCs at a high concentration. Almost 1 billion PBMCs can be recovered from this low-volume blood product. - Leukopak
Leukopaks have a higher volume than leukoreduction system chambers and are used when isolating white blood cells. The typical concentration of PBMCs in a leuopak is about 5 ×107 cells/mL.
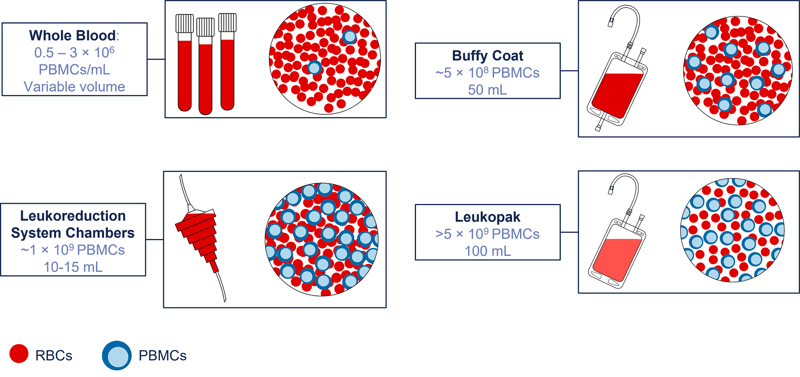
Figure 3: Clinical sources of PBMCs include whole blood, buffy coat, leukoreduction system chambers, and leukopak samples.
Methods for isolating PBMCS
The most popular technology for PBMC isolation is the Ficoll Overlay technique, or density gradient medium (DGM) centrifugation, which was developed in the 1960s. However, this method can be time-consuming and subject to inter-operator variability. Recent technological advances to address these issues have improved the methodology for use in clinical research.
- Frit Barrier
Tubes containing a frit barrier provide better separation of the blood layers during the centrifugation step. Use of a frit barrier decreases the overall processing time and improves standardization - you will probably recognize systems such as Accuspin, SepMate, and LeucoStep. One drawback of the frit barrier is that it can result in reduced PBMC yields compared with the standard Ficoll Overlay technique. - Cell Preparation Tubes
Sometimes clinical trial protocol specifications are time-critical, and it is more convenient to have partial on-site processing of PBMCs. To provide quicker and easier PBMC isolation, cell preparation tubes (CPT) contain anticoagulant plus a gel product that aids blood layer separation. CPT tubes can be centrifuged on site and then shipped to the laboratory for the final isolation steps. However, if blood quality is poor (e.g., if there is hemolysis or coagulation present) effective PBMC isolation cannot be achieved. - Immunomagnetic Cell Separation
Immunomagnetic cell separation (column-based or column-free) is a method for providing high-yield, high-purity PBMCs. This technique can also be used to enrich a sample for specific immune cell subsets. These platforms - which include MACSprep Kits and EasySep Kits - allow rapid, effective isolation of cells. Unfortunately, this technique is expensive and more difficult to use with larger sample sizes.
| Density Gradient Medium | Frit Barrier | Cell Preparation Tubes | Immunomagnetic Cell Separation | |
| Technology | Ficol Overlay | Accuspin, SepMate, LeucoSep | CTP Vacutainers with different anti-coagulants | MACSprep kits, EasySep kits |
| Advantages | Standard | Easier to standardize, reduced processing time | On-site processing, significantly reduced processing time | Isolation of cell subsets, quicker |
| Disadvantages | Intra- and iter-operator variability, time-consuming | Reduced PBMC yields compared to standard | Higher possibility for contamination | More expensive, less available |
Table 2: Comparison of PBMC processing options and their advantages/disadvantages.
Counting and freezing PBMCs
The final step in your isolation process is to assess PBMC quality. The viability and quantity of PBMCs in your isolate can be estimated via manual cell counting. Using trypan blue exclusion (stains dead cells) to visualize, a high-quality PBMC isolate, when processed fresh, has > 90% viability and a low concentration of erythrocytes and granulocytes.4
However, manual cell counting can be time-consuming and result in variation between operators. Automated counters provide an alternative standardized approach that is quicker to perform and often the preferred method for clinical research studies.5-6
A key advantage of PMBCs is that they can be stored in a viable condition long-term. If you are looking to preserve your PBMCs, controlled-rate PBMC freezing will give the best results. This process involves transferring samples into short-term cryogenic storage for 1-3 days (e.g., a -80°C freezer) before long-term cryogenic storage (e.g., vapor phase liquid nitrogen). We have included protocols for manually counting and cryopreserving your PBMCs in this blog article.
Troubleshooting poor-quality PBMCs
There are many factors that can impact the quality of PBMCs and any downstream biological investigation. We have grouped the most common into categories below so that you can optimize your PBMC isolation protocols.
Sub-optimal sample collection
The venepuncture process is crucial. Poor phlebotomy technique, incomplete filling of sample tubes, incorrect needle gage, time of collection, and the type of tubes/anticoagulant used can all affect sample quality. Different anticoagulants prevent clotting in different ways (e.g., some act as calcium chelators, while others bind to thrombin) -as a result, some anticoagulants may affect end-point analyses. For biomarker studies, a blood sample taken in the morning may differ from one taken in the evening. The size of the needle gage may be too small or large and affect the flow and activation of cells. Care should be taken to consider any aspect of the collection process that could affect your cells and biomarkers of interest.7
Delay between collection and processing
According to a recent study, the time between sample donation and processing is important for the yield and viability of PBMCs isolated (figure 5).7 Regardless of the isolation methodology used, a freshly processed blood sample will generate a high percentage of T-cells and a low percentage of granulocytes. If the processing time is delayed, the sample ages, cells begin to die, and granulocytes become activated (increasing their relative percentage). Since granulocytes can negatively affect PBMC isolates in several ways - including oxidative stress, contamination of the PBMC fraction, and interference with downstream analysis - it is critical to prevent delays between sample collection and processing.
-1.png?width=800&name=Betsou%20F%20et%20al.%20Curr%20Pathobiol%20Rep%207%2c%2017%E2%80%9327%20(2019)-1.png)
Figure 5: Delay before processing can increase the percentage of granulocytes in a sample. Source: Betsou F et al. Curr Pathobiol Rep 7, 17–27 (2019)7
Incorrect processing temperature
Another important factor that can affect sample quality is storage and transport temperature. It is important to perform PBMC isolation between 18-25°C prior to cryopreservation. A centrifuge with adjustable temperature is recommended if you think that this is affecting sample quality.
Supplemented washing reagents
Low ionic strength, calcium/magnesium free DPBS is generally the wash buffer of choice for protocols. If you decide to supplement this washing reagent with BSA or FBS to improve yield, note that these serums can affect some downstream applications.
Poor cryopreservation technique or reagents
A robust freezing protocol is important for preventing severe cell damage, osmotic injury, and intra- or extra-cellular water crystal formation. Cells should be frozen in cryopreservation vials in cryoprotectant with freezing gradually applied at a rate of ~1°C/min. The DMSO used during cryopreservation should be high-quality with low levels of endotoxins. This also applies to any cell culture grade DMSO, or animal/human serums used in the culture or storage of PBMCs. Cryopreservation media that is optimized for PBMC isolation is commercially available if required by clinical protocols.
Outsourcing work to a PBMC company
If you have a large number of samples to process or lack internal resources to perform PBMC isolation in-house, you may want to consider outsourcing your cell isolation and enrichment requirements to a central laboratory service provider. To find a reputable partner, you should consider the following:
- Relevant sample handling and processing experience
- 24/7 sample receipt and processing capacity
- Location of facilities
- Quality and project management systems
If you need further downstream processing, it is also useful to consider whether the company offers additional services to meet your project requirements. At REPROCELL, we can access a wide range of donors, derive both human PBMCs and CBMCs, and provide molecular analysis for an additional fee. You can find out more information about our whole blood processing services here or in the webinar below.
PBMC processing protocols
- Protocol for buffy coat preparation from whole blood
- Protocol for PBMC isolation from buffy coat samples
- Isolating PBMCs from whole blood using density gradient centrifugation
References
- Kolundzic N. Customized Central Laboratory Services for Support of Global Clinical Trials: PBMC Processing REPROCELL Webinar Series (2022).
- Bittersohl et al. Intracellular concentrations of immunosuppressants. Personalized Immunosuppression in Transplantation (2016).
- Bøyum et al. Isolation of mononuclear cells and granulocytes from human blood (Paper IV). Scand J Clin Lab Invest, 97, 77–89 (1968).
- Sahaf et al. Redox Cell Biology and Genetics Part B. Methods in Enzymology (2002).
- Virgilio et al. Tumour Immunology and Immunotherapy - Molecular Methods. Methods in Enzymology (2012).
- Mahajan et al. Nanomedicine. Methods in Enzymology (2012).
-
Betsou F et al. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Current Pathobiology Reports 7 pp 17–27 (2019).


.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



