Preparation of a buffy coat from whole blood samples precludes many downstream applications.1 During processing, whole blood is centrifuged to form distinct blood fractions. If you are looking for a simple protocol to use to isolate the buffy coat from your blood samples, our scientists have outlined the key steps in this article.
Why is the buffy coat important?
The buffy coat is a fraction of whole blood that is rich in white blood cells (WBCs) and is named after its "buff-like" color.2 Researchers may want to isolate cells contained in the buffy coat for a number of reasons. These include, but are not limited to downstream processes such as:1,2
- Peripheral blood mononuclear cell (PBMC) isolation (you can access a protocol for this process here)
- T-cell depletion
- Tumour cell purging
- DNA and RNA extraction
Buffy coat isolation protocol
It takes no longer than 30 minutes to isolate the buffy coat using a standard blood bank centrifuge.2 The below protocol describes a density gradient centrifugation methodology that retains 80% of nucleated blood cells while reducing the number of red blood cells (RBCs) by 80%.2 Please note that this methodology is not recommended where downstream chemical or immunological purging would be affected by RBC contamination. It requires a whole blood sample pre-treated with an anticoagulant.2,3
- Pipette the blood sample into a 50 mL conical tube at a ratio of 1:1 with the recommended media.
- Invert to mix gently
- Centrifuge the sample(s) for 10 minutes at 800 ×g.
- Remove the sample from the centrifuge carefully, so as not to disturb the distinct blood fractions.
- Pipette the buff-colored layer, being careful not to disturb the other blood components, and transfer it into another centrifuge tube.
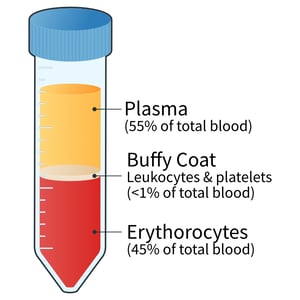
- The buffy-coat resides at the plasma-RBC junction (figure 1)
- Further processing can be used to enrich the buffy-coat and remove residual RBCs
Blood layers after centrifugation
There are several ways to quantify the number and distribution of cells in your buffy coat sample.4 For example, automated cell counting can be achieved using impedance counters and laser flow cytometry. The former uses changes in electrical impedance to estimate cell size and quantity, while the latter uses interruptions in light signals. Otherwise, manual techniques such as quantitative buffy coat analysis (QBC) and capillary tube methods can be used if automated tools are unavailable.
Figure 1: Blood layers after centrifugation. Following centrifugation, the blood components are separated into distinct layers based on their density. The white blood cell (WBC) rich buffy coat can be found at the erythrocytes-plasma junction, composing <1% of the total blood volume. Cells in this layer are denser than plasma components but less dense than the RBCs at the bottom of the tube.
References
- Bittersohl et al. Intracellular concentrations of immunosuppressants. Personalized Immunosuppression in Transplantation (2016).
- Cottler-Fox et al. Collection and processing of marrow and blood hematopoietic stem cells. Hematopoietic Stem Cell Transplantation in Clinical Practice (2009).
- Bidmon et al. Tumor Immunology and Immunotherapy - Cellular Methods Part A. Methods in Ezymology (2020).
- Harvey. Hematology Procedures. Vetinary Hematology (2012).
Editors note: This protocol is for guidance only, based on freely available information and typical protocols used in labs worldwide. REPROCELL is not responsible for the results of any work using this protocol(s).


.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



