Peripheral Blood Mononuclear Cells (PBMCs) are contained within the buffy coat alongside platelets, leukocytes, and granulocytes. To isolate PBMCs from the buffy coat, density gradient centrifugation can be used.1
PBMCs may be removed from the buffy coat using manual or automated methods, with or without density-gradient materials such as albumin, Ficoll, and Percoll.2 These hydrophilic colloids (polymers formed by copolymerization of sucrose and epichlorohydrin or polyvinylpyrrolidone-coated colloidal silica) remove essentially all RBC and myeloid elements, leaving only mononuclear cells.1,2
If you are looking to isolate PBMCs from whole blood samples instead, we have outlined a protocol for this here.
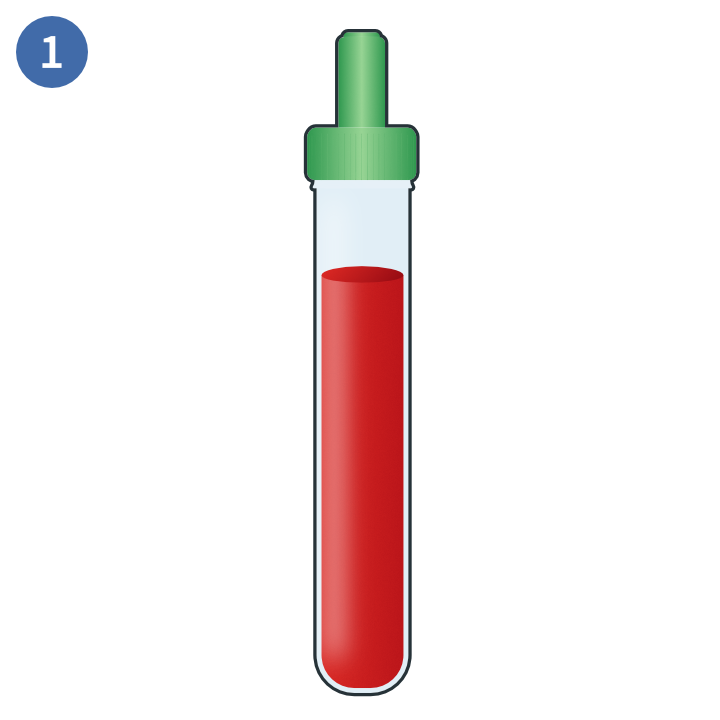
Whole blood sample
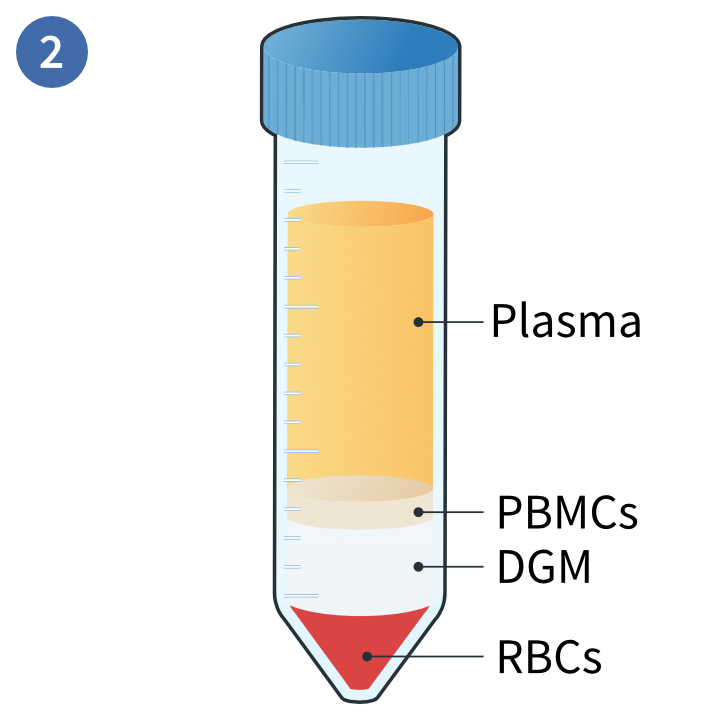
Separation of distinct layers
 Collection of PBMCs
Collection of PBMCs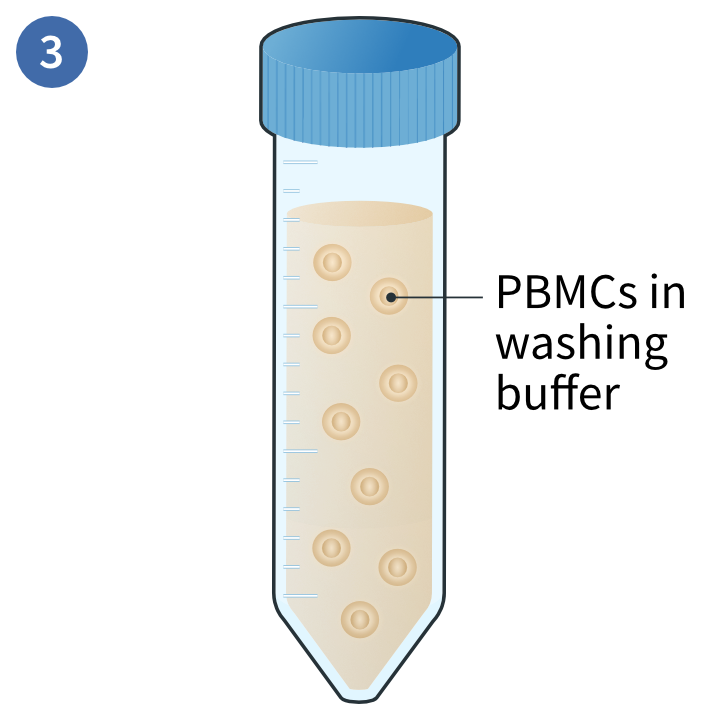
Isolation of PBMCs
PBMC isolation from buffy coat samples
- Collect your buffy coats one day prior to the assay.
- Isolate the buffy coat from whole blood samples, or procure them from a biorepository.
- Read our protocol for buffy coat isolation from whole blood here
- Dilute the buffy coat with PBS 1:1 in a 50 mL conical tube and gently mix by inversion
- Use a separate conical tube per donor
- Dispense 15 mL of room temperature density gradient media (DGM) into a 50 mL conical tube.
- Pour 35 mL of the blood/PBS on top of the DGM
- Avoid mixing them so that you have 2 separate phases
- Centrifuge the tubes at 900 × g for 22 minutes at room temperature.
- Add 10 mL PBS into a 15 mL tube and transfer the PBMC layer from the centrifuged tubes without affecting the interphase or aspirating the DGM.
- Invert the tubes gently to mix
- Centrifuge the 15 mL tubes containing the PBMCs-PBS mixture at 250 ×g for 5 minutes.
- Dispose of the supernatant
- Resuspend the pellet in PBS and centrifuge at 250 × g for 5 minutes at room temperature.
- Dispose of the supernatant
- Repeat washing steps 6-7 if the supernatant is still cloudy (optional)
- Resuspend the washed pellet at the desired concentration for downstream use
- If you need help counting your cells, we have outlined a protocol in the next section of this article
PBMC cell count protocol
In this protocol3, we will describe how you can manually estimate the number of PBMCs in your sample using trypan blue and a hemocytometer or similar chamber.
- Prepare a 1:1 dilution of your cells with 0.4% trypan blue in a 2 mL tube.
- Pipette the solution up and down to mix
- Incubate cells in this mixture for 2-3 minutes.
- Prepare the hemocytometer while the cells are incubating with the mixture
- Transfer 10 µL into the counting chamber and count the number of alive (non-blue) cells and dead cells (blue) using a light microscope.
- Use this number to estimate the total number of viable cells in the original sample
References
- Bittersohl et al. Intracellular concentrations of immunosuppressants. Personalized Immunosuppression in Transplantation (2016).
- Cottler-Fox et al. Collection and processing of marrow and blood hematopoietic stem cells. Hematopoietic Stem Cell Transplantation in Clinical Practice (2009).
- Bidmon et al. Tumor Immunology and Immunotherapy - Cellular Methods Part A. Methods in Ezymology (2020).
Editors note: This protocol is for guidance only, based on freely available information and typical protocols used in labs worldwide. REPROCELL is not responsible for the results of any work using this protocol(s).


.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



