Drug Discovery Phases
Clinical trials for Investigational New Drugs (IND) are one of the biggest costs associated with drug development. And yet, up to 90% of all INDs fail at some phase during clinical trials. A lack of translational preclinical models that accurately mimic human physiology is partly to blame.
Demonstrating human efficacy and safety at an early stage of development represents enormous commercial value to your research programs. For a fifth of drugs tested in human models, researchers rethink their clinical strategy to prevent failure. It is estimated that preclinical human testing has saved Pharma more than $55 billion in research funding so far.
At REPROCELL, we provide access to human biospecimens and iPSC-derived models that de-risk your drug discovery programs. No other company has this unique combination of expertise in preclinical human assays.
Target Identification
Using human tissue samples in your preclinical research can accelerate target identification. As home to one of the largest human tissue networks, we can provide access to over 600,000 samples linked to 120,000 patients. Diseased and normal tissue are available via our network, including matched sample sets. Our BioServe network of partner organizations provides broader access to rare samples and the ability to source material specific to your research needs.
Each human tissue sample is provided with:
- Detailed demographic information
- Gold standard clinical diagnostic information
- Complete drug history, including ACE
- A full pathology report, including H&E slides
- Complete phenotypic data
Patient recruitment and tissue collections:
- Governed by IRB protocols and HIPAA regulations
- Ethically collected and broadly consented
- Sample data anonymized from original consents

Our global biorepository contains more than 600,000 human biospecimens from over 120,000 donors.

REPROCELL's biospecimen partner network provides broader access to rarer human tissue samples.

The ability to source material specific to your research needs can be achieved through our prospective collections.
Target Validation and Lead Identification
If you are seeking functional validation of disease targets or lead identification, this can be achieved via our custom drug discovery projects. We can investigate your target's therapeutic potential using patient derived explants (PDE) or patient-specific iPSC-derived cells to provide preclinical human data.
Induced pluripotent stem cells
Our induced pluripotent stem cells (iPSCs) are created by reprogramming patient samples using StemRNA™ 3rd Gen RNA Reprogramming technology. Relevant patient samples can be sourced from our network of clinical centers, or you can send us the primary materials to be reprogramed. Resultant iPSC clones are validated for pluripotency, authenticity, and genetic integrity.
If you require differentiated cell types, our scientists can prepare mature cells for experimentation. Unlike primary cells, which can possess variable genetic backgrounds, our iPSC-derived cells have strong lot-to-lot performance consistency. We also offer advanced genome editing* which is ideal for challenging projects, such as SNPs or isolating homo-heteroallelic clones.
*The modified cells are developed, manufactured, or supplied by GenAhead Bio Inc. under license from ERS Genomics Limited.
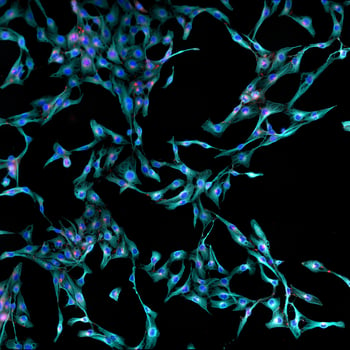
Tissue procurement and isolation of primary cells
Reprogramming of cells using foot-print free mRNA technology
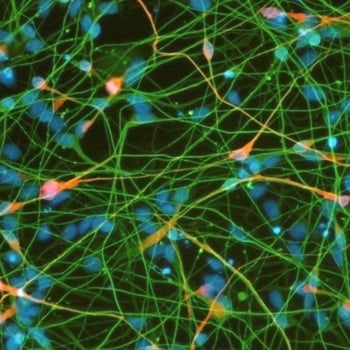
Differentiation of cells into somatic cell types e.g. neurons
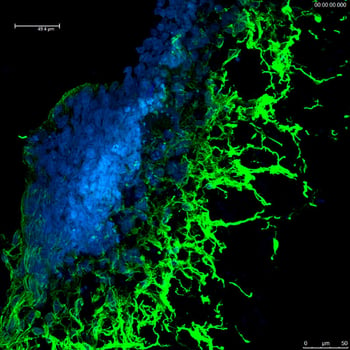
Advanced genome editing using CRISPR-SNIPER technology*
3D Bioengineered Tissue Models
Three-dimensional (3D) cell models are becoming increasingly popular in disease modeling and drug discovery. Our organotypic 3D Bioengineered Tissue Models use Alvetex technology to provide the highest standards of clinical translatability. A range of cell types can be cultured in these 96-well plates; we typically use primary cells or iPSC-derived neurons for our client projects.
What is Alvetex?
Alvetex is a highly porous, inert scaffold made from cross-linked polystyrene that is 200μm thick. Because Alvetex maintains human physiology, cell viability, and longevity, cells cultured in this scaffold deliver more in vivo-like results than traditional 2D monolayer cultures. Alvetex allows perfusion in three dimensions and provides simple histology, imaging, and gene expression endpoints.
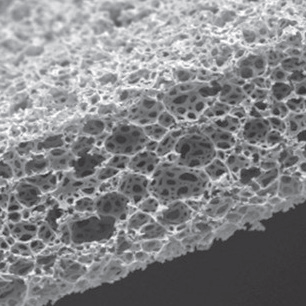
Scanning electron microscope image of Alvetex Scaffold
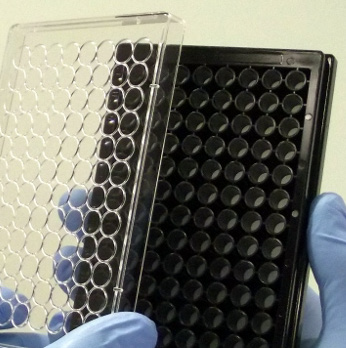
96 well plate format available for high throughput assays
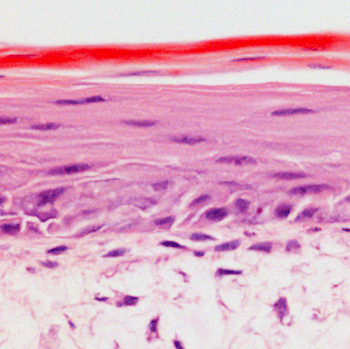
Co-culture of keratinocytes and dermal fibroblasts
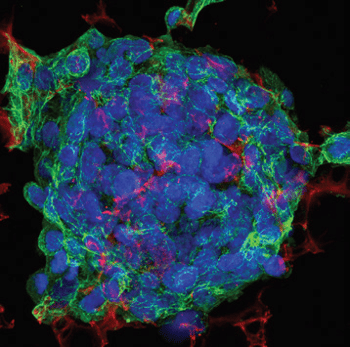
Triple fluorescent staining of HepG2 cells grown in 3D on Alvetex
Lead Optimization
Fresh tissue testing is one of the most translational methods for investigating test compound effectiveness and absorption at a preclinical stage. By generating human data before expensive clinical studies, you can greatly increase the predictability of your drug development and make ‘go’ or ‘no-go’ decisions earlier. Human data adds significant value for investors and is invaluable when repositioning compounds.
At REPROCELL, we are the only GLP-accredited human tissue testing laboratory. We offer a range of models across all therapeutic areas and have a dedicated team who design and deliver these specialized projects. Both healthy and diseased biospecimens are available for testing in our validated assays. The flow chart below shows a typical lead optimization project; note that the tissue type and endpoint are highly flexible.
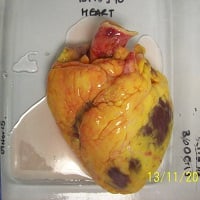
1. Fresh tissues are transported to the laboratory

2. Scientist isolates the structure of interest e.g. small blood vessels

3. Living tissues are exposed to test articles
The benefits of human tissue testing
Efficacy data from living human tissue is a powerful way to demonstrate the value of your compounds. It adds commercial value by reducing the risk of phase II failure, de-risks development by identifying lead compounds, and provides target population data at an early stage. Assessing the efficacy of your drug candidate in human tissues not only increases confidence but allows you:
- To identify the drug target and measure biological response in phenotypically relevant tissues
- To compare activation of potential target and functional response
- To explore differences in drug response between patients and relate response to clinical histories
- To relate the functional activation of the target to donor genotype

A fresh human skin sample, used to create punch biopsies for our organotypic assays.
Pre-clinical Safety
Through our wide range of human tissue and iPSC-derived assays, we can provide human safety data for your compound early in the development cycle. Human tissue assays can be used to assess the safety of your compound in abundant tissue types, whereas our iPSC-derived disease models can be used for neuronal research.
Human tissue assays
The benefits of undertaking safety testing in human tissues are many. When carried out in conjunction with the ICH S7A core battery, our data can provide an unparalleled indication of clinical safety and even reduce the number of animals required for in vivo experimentation. In addition to reducing animal testing, human safety data adds significant value for investors and potential partners. Routine safety assays conducted by REPROCELL include:
| Condition | Tissue | Technique |
| Blood pressure | Human subcutaneous resistance arteries | Vessel diameter – wire myography |
| Myocardial infarction or angina | Human coronary artery | Vessel diameter – wire myography |
| Diarrhea or constipation | • Human colon • Human gall bladder |
• Fluid transport – Ussing chambers • Gut motility – Organ baths • Gall bladder contractility – Organ baths |
| Cardiac contractility | Human ventricular trabeculae | Electrical field stimulation – Organ baths |
iPSC-derived Neuron Models
REPROCELL has developed differentiation protocols for normal and disease-model neurons including astrocytes and sensory, motor, and dopaminergic neurons. Our robust manufacturing workflow produces mature cell types that are electrophysiologically responsive, organotypic, and display low lot-to-lot variation
Our human neurons can be used to assess a range of end-points, including neurite outgrowth, electrical activity, and protein expression. With decades of experience in custom cell model development, we can develop custom differentiation protocols, or use existing protocols to create your desired disease model system.
Animal and human comparison studies
Comparisons between human and animal data are often vital, as animal data does not always translate to humans. For example, human intestinal tissue more accurately predicts in vivo bioavailability and drug metabolism than animal tissues. REPROCELL can conduct comparative pharmacology studies to compare the behavior of your drug between humans and animals. This allows you:
- To investigate unexpected observations during clinical trials
- To investigate observations from in vivo animal models
- To investigate known species differences for the target/mechanism
- To investigate requests from regulators (all projects are available as GLP studies if required)

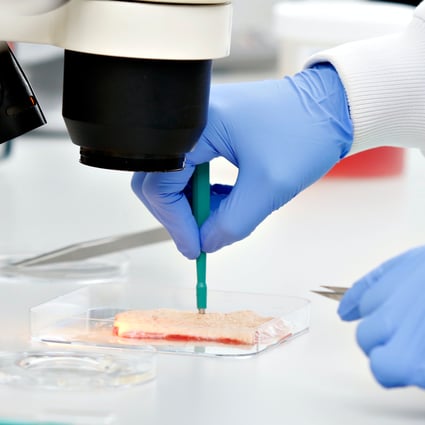
One of our scientists creating punch biopsies from a skin sample resection.
Clinical Trials
By returning to ex vivo testing during human trials, you can investigate adverse clinical effects (ACE) and troubleshooting clinical problems. Using human fresh tissue testing during this stage, you can:
- Obtain independent verification of your clinical results
- Identify pathways responsible for the ACE
- Determine whether species differences are responsible for failure to predict the ACE
- Rescue your compound from clinical failure by identifying drugs that can combat the ACE

Our human tissue experts operating the wire myograph systems in our Glasgow laboratory.
Making an inquiry
- Contact our experts using the form below, to discuss a protocol designed specifically for your needs
- We will send you a fully-costed proposal and timeline
- At the end of your project, REPROCELL will provide you with raw data and a written report
- Tissues or test compounds can be returned to you for further testing




![Wire myography: the ultimate guide [protocol included]](https://www.reprocell.com/hs-fs/hubfs/REPROCELL-04.06.18_0163.jpg?width=756&height=425&name=REPROCELL-04.06.18_0163.jpg)