Skin Biopsies from Psoriasis Donors
Drug Discovery Assay – reference number: B119
| Assay type: | Skin |
| Tissue: | Skin disease biopsies |
| Target: | Study dependent |
| Control compound: | Betamethasone |
| Study type: | Ex vivo cultures |
| Functional endpoint: | Inflammation |
Assay Description
This experiment assesses whether test articles cause a reduction in inflammatory gene expression release in psoriasis skin biopsies, with Betamethasone as a reference compound. It uses clinical skin biopsies obtained from psoriasis donors to explore test article effect on gene expression via rtPCR.
Testing Information
Introduction
The specific results that will be provided are on the anti-inflammatory effect of test articles across psoriasis skin biopsies.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in deionized water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
We suggest test conditions are assessed in singlet across 5–10 donors.
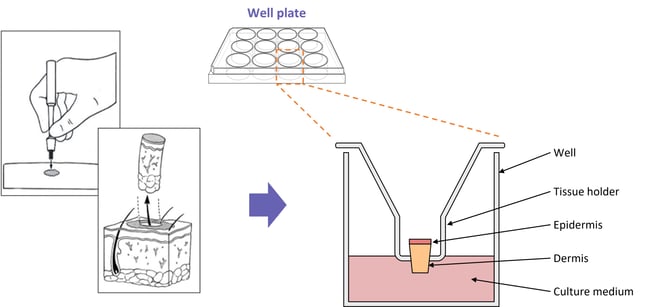
Diagram showing a typical setup for our skin disease explant assays
Study Outline
Rationale and Experimental Design
Full-thickness skin biopsies are obtained from our clinical networks. Biopsies are taken at a size of 3mm2 and then transferred into fortified media, leaving the epidermis exposed to air. Biopsies are incubated in optimum conditions for a maximum of 24 hrs, then cultured in the presence of the test article for a further 24 hrs.
An example of the conditions assessed for 1 test article is detailed below (it is recommended a minimum of 5–10 donors should be used for each condition):
- Control (diseased biopsy)
- Test article (treated, disease biopsy)
- Control 2 (non-diseased biopsy)
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue.
Analysis
Recommended end point analysis includes gene expression, RNA sequencing or immunohistochemistry.
Data from Psoriasis skin biopsies
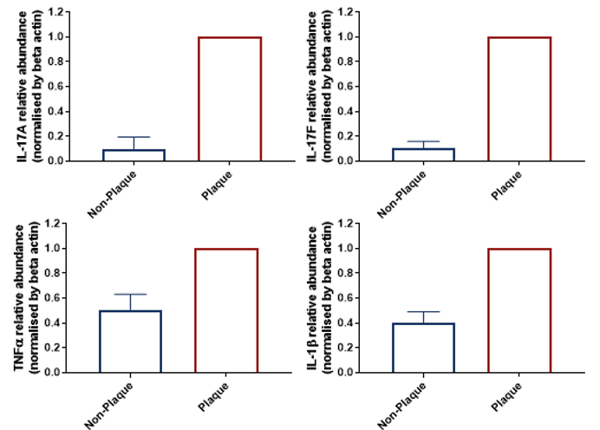
Characterization by qRT-PCR: Gene expression profiles are different between non-plaque and plaque biopsies for key psoriasis genes.


.jpg?width=756&height=425&name=Untitled%20design%20(5).jpg)