Recombinant human vitronectin protein
QK120
Brand: Qkine
Vitronectin protein is widely used in stem cell culture. It provides a defined environment that supports the maintenance of pluripotency and is suitable for feeder-free culture, expansion, differentiation, and reprogramming of stem cells.
Qkine vitronectin is a high-purity animal origin-free recombinant protein with a molecular weight of 47.8 kDa. It is carrier-free and protein tag-free, ensuring exceptional lot-to-lot consistency and compatibility with translational studies and iPSC-based model production for screening. Qkine recombinant vitronectin protein is ultra high-purity with exceptionally low residual endotoxins making it suitable for the reproducible culture of stem cells, primary cells, organoids, and use in sensitive neural differentiations.
Currency:
| Product name | Catalog number | Pack size | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|
| Recombinant human vitronectin protein, 500 µg | QK120-0500 | 500 µg | (select above) | $ 165.00 | £ 120.00 | € 141.00 |
| Recombinant human vitronectin protein, 5000 µg | QK120-5000 | 5000 µg | (select above) | $ 685.00 | £ 500.00 | € 584.00 |
Note: prices shown do not include shipping and handling charges.
Qkine company name and logo are the property of Qkine Ltd. UK.
Alternative protein names
VTN-N
S-protein
Serum-spreading factor, V75
Species reactivity
human
species similarity:
mouse – 74%
rat – 76%
porcine – 74%
bovine – 72%
Frequently used together:
- Recombinant FGF2-G3 (145 aa) protein (QK052)
- Recombinant FGF2-G3 (154aa) protein (QK053)
- Recombinant human TGF-β1 PLUS™ protein (QK010)
- Recombinant human TGF-β3 protein (QK054)
- Recombinant human VEGF 165 protein (QK048)
- Recombinant human PDGF-BB protein (QK044)
- Recombinant human IGF-1 protein (QK047)
- Recombinant human PDGF-AA protein (QK043)
Summary
- Highly pure recombinant human vitronectin protein (truncated) (UniProt: P04004)
- 47.8 kDa (monomer)
- >98%, by SDS-PAGE quantitative densitometry
- Expressed in E. coli
- Animal origin-free (AOF) and carrier protein-free
- Manufactured in Qkine's Cambridge, UK laboratories
- Lyophilized from PBS, mannitol and TCEP
- Resuspend in sterile-filtered water at 1 mg/ml, add carrier protein if desired, prepare single use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term).
Featured applications
- Induced pluripotent and embryonic stem cell differentiation and maintenance
- Differentiation of human pluripotent stem cells towards extra-embryonic endoderm, mesenchymal, neural lineages, and chondrocytes
- Promotion of cell adhesion and migration
- Organoid growth and proliferation
- Stimulation of angiogenesis and vascular network development
- Fully defined, animal-free protocols for translational studies and preclinical stem cell production
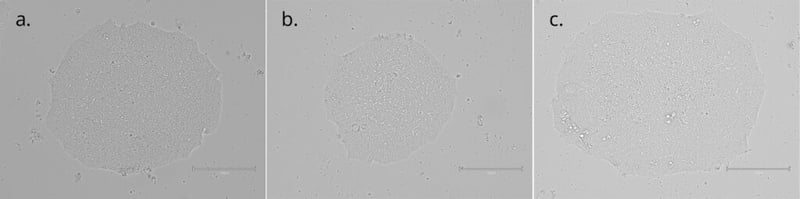
Imaging of colonies grown on Qkine vitronectin coated plates in E8-like media retained their highly preserved morphological appearance. (A) after initial seeding and 3 days in culture; (B) After 1 passages and 7 days in culture; (C) After 3 passages and 14 days in culture (scale bar = 150µm). 6-well plates were coated with 5 µg/ml Qk120 vitronectin protein lot #204660.
Preparation of cell culture plates with Qkine ultra-high quality vitronectin (Qk120)
- Briefly centrifuge vial containing vitronectin Qk120 to ensure all lyophilized protein is collected at the bottom of the vial.
- Resuspend in 500 ml of MilliQ water to make a 1 mg/ml stock and lightly agitate the tube to ensure everything has fully reconstituted.
- Per 6-well plate required, dilute 30 µl of the 1 mg/ml in 6 ml of phosphate buffered saline (PBS) without MgCl and CaCl to make 5 µg/ml solution.
- Coat each well of 6-well plate with 1 ml per well of 5 µg/ml vitronectin for at least two hours at 37°C.
- Adjust volume of 5 µg/ml vitronectin per well for different area plate clusters.
- 12-well plate add 500 µl
- 24-well plate add 250 µl
- 96-well plate add 100 µl
- Aliquot the remaining resuspended 1 mg/ml vitronectin into appropriately sized single use aliquots and store at -80°C.
Further quality assays
- Mass spectrometry, single species with the expected mass
- Endotoxin: <0.005 EU/μg protein (below the level of detection)
- Recovery from stock vial: >95%
Protein background
In tissue engineering and regenerative medicine, vitronectin-coated scaffolds and biomaterials enhance cell attachment, proliferation, and differentiation, contributing to the development of functional tissue constructs [15-16]. Additionally, vitronectin is used to prepare cells for cell therapy, ensuring their viability and functionality upon transplantation.
Vitronectin is particularly important for optimizing culture conditions in the manufacturing of serum-free media, compensating for the lack of adhesion-promoting factors typically provided by serum and supporting cell growth under defined conditions [7, 17]. The use of recombinant vitronectin protein ensures batch-to-batch consistency, which is critical for reproducibility in experimental and clinical applications.
Structurally, vitronectin is a multifunctional glycoprotein with a molecular weight of approximately 75 kDa. It comprises several domains, including the somatomedin B domain at the N-terminal, which is important for binding to the urokinase receptor and stabilizing the inactive form of plasminogen activator inhibitor-1 (PAI-1). The RCL (Ricinus communis agglutinin-like) domain is crucial for heparin binding, while the hemopexin-like repeats facilitate interactions with integrins and other ECM components [2,6,18]. Vitronectin can undergo conformational changes that expose or hide specific binding sites, enabling it to interact with a wide range of ligands and receptors.
In summary, Vitronectin plays a crucial role in various applications, particularly in stem cell and organoid applications, by providing a defined and supportive environment for stem cell maintenance, differentiation, and reprogramming. Its use enhances the reproducibility and efficiency of several cell cultures, making it a valuable tool in both research and therapeutic contexts.
Background references
- Schvartz, I., Seger, D. & Shaltiel, S. Vitronectin. The International Journal of Biochemistry & Cell Biology 31, 539–544 (1999). doi: 10.1016/s1357-2725(99)00005-9
- Feldinghabermann, B. & Cheresh, D. Vitronectin and its receptors. Current Opinion in Cell Biology 5, 864–868 (1993). doi: 10.1016/0955-0674(93)90036-p
- Hurt, E. M. et al. Identification of Vitronectin as an Extrinsic Inducer of Cancer Stem Cell Differentiation and Tumor Formation. Stem Cells 28, 390–398 (2010). doi: 10.1002/stem.271
- Jang, Y.-C., Tsou, R., Gibran, N. S. & Isik, F. F. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 127, 696–704 (2000). doi: 10.1067/msy.2000.105858
- Preissner, K. T. The role of vitronectin as multifunctional regulator in the hemostatic and immune systems. Blut 59, 419–431 (1989). doi: 10.1007/BF00349063
- Madsen, C. D. & Sidenius, N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. European Journal of Cell Biology 87, 617–629 (2008). doi: 10.1016/j.ejcb.2008.02.00
- Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8, 424–429 (2011). https://pubmed.ncbi.nlm.nih.gov/21478862/
- Hasanuzzaman, M., Kutner, R., Agha-Mohammadi, S., Reiser, J. & Sehgal, I. A doxycycline-inducible urokinase receptor (uPAR) upregulates uPAR activities including resistance to anoikis in human prostate cancer cell lines. Mol Cancer 6, 34 (2007). doi: 10.1186/1476-4598-6-34
- Kaini, R. R., Shen-Gunther, J., Cleland, J. M., Greene, W. A. & Wang, H.-C. Recombinant Xeno-Free Vitronectin Supports Self-Renewal and Pluripotency in Protein-Induced Pluripotent Stem Cells. Tissue Engineering Part C: Methods 22, 85–90 (2016). doi: 10.1089/ten.TEC.2015.0180
- Rowland, T. J. et al. Roles of Integrins in Human Induced Pluripotent Stem Cell Growth on Matrigel and Vitronectin. Stem Cells and Development 19, 1231–1240 (2010). doi: 10.1089/scd.2009.0328
- Sohi, A. N. et al. Synergistic effect of co-immobilized FGF-2 and vitronectin-derived peptide on feeder-free expansion of induced pluripotent stem cells. Materials Science and Engineering: C 93, 157–169 (2018). doi: 10.1016/j.msec.2018.07.072
- Ahn, S. et al. Engineering fibronectin-templated multi-component fibrillar extracellular matrices to modulate tissue-specific cell response. Biomaterials 308, 122560 (2024). https://doi.org/10.1016/j.biomaterials.2024.122560Zhu, Z. et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell 26, 187-204.e10 (2020). doi: 10.1016/j.stem.2019.11.016
- Wijler, L. A. et al. Onward Spread from Liver Metastases Is a Major Cause of Multi-Organ Metastasis in a Mouse Model of Metastatic Colon Cancer. Cancers 16, 1073 (2024). doi: 10.3390/cancers1605107
- Juhásová, J. et al. Osteogenic Differentiation of Miniature Pig Mesenchymal Stem Cells in 2D and 3D Environment. Physiol Res 559–571 (2011) doi:10.33549/physiolres.932028. doi:10.33549/physiolres.932028
- Papo, N., Silverman, A. P., Lahti, J. L. & Cochran, J. R. Antagonistic VEGF variants engineered to simultaneously bind to and inhibit VEGFR2 and α v β 3 integrin. Proc. Natl. Acad. Sci. U.S.A. 108, 14067–14072 (2011). doi: 10.1073/pnas.1016635108
- Richards, S., Leavesley, D., Topping, G. & Upton, Z. Development of Defined Media for the Serum-Free Expansion of Primary Keratinocytes and Human Embryonic Stem Cells. Tissue Engineering Part C: Methods 14, 221–232 (2008). doi: 10.1089/ten.tec.2007.0428
- Jensen, J. K., Wind, T. & Andreasen, P. A. The vitronectin binding area of plasminogen activator inhibitor‐1, mapped by mutagenesis and protection against an inactivating organochemical ligand. FEBS Letters 521, 91–94 (2002). doi: 10.1016/s0014-5793(02)02830-2
FAQ
What is vitronectin?
A glycoprotein found in the extracellular matrix (ECM) that plays critical roles in cell adhesion, migration, wound healing and regulation of immune response.
Where is vitronectin found?
Vitronectin is found in the extracellular matrix, blood plasma and various tissues.
Is vitronectin a cytokine?
Vitronectin is not a cytokine but it signals through integrin receptors to support maintenance of pluripotency during iPSC culture.
What does the vitronectin gene do?
Vitronectin gene encodes vitronectin protein, which is a component of the extracellular matrix.
What does vitronectin bind to?
Vitronectin binds to integrins, heparin and heparan sulfate proteoglycans, plasminogen activator inhibitor-1, complement system.
What is the function of the vitronectin receptor?
The vitronectin receptor facilitates cell adhesion, cell migration and initiates signaling processes that promotes cell survival, proliferation and differentiation.
How is vitronectin used in cell culture?
Vitronectin is widely used in stem cell culture. It provides a defined environment than supports maintenance of pluripotency and is suitable for feeder-free culture, maintenance, differentiation, and reprogramming of stem cells.