Ion Channel Function in Isolated Healthy Human Gastrointestinal Mucosa (Chloride Channels)
Drug Discovery Assay – reference number: B094
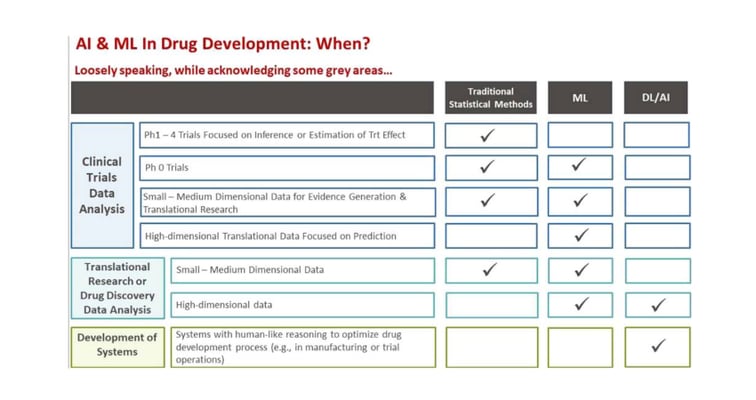
Overview
| Assay type: | GI tract |
| Tissue: | Human gastrointestinal mucosa (healthy) |
| Target: | Chloride channels |
| Control compound: | Cholera toxin |
| Study type: | Ussing chamber |
| Functional endpoint: | Ion channel function |
Assay Description
This assay assesses whether test articles cause a change in short circuit current across healthy human gastrointestinal mucosa. It is possible to assess ion channel function across multiple regions of the GI system.
The intestines are essential for the digestion of food and the absorption of fluid and electrolytes. Changes in the anatomy or function of the intestines can lead to disorders such as Irritable Bowel Syndrome (IBS), Crohns, Ulcerative Colitis and diarrhoea.
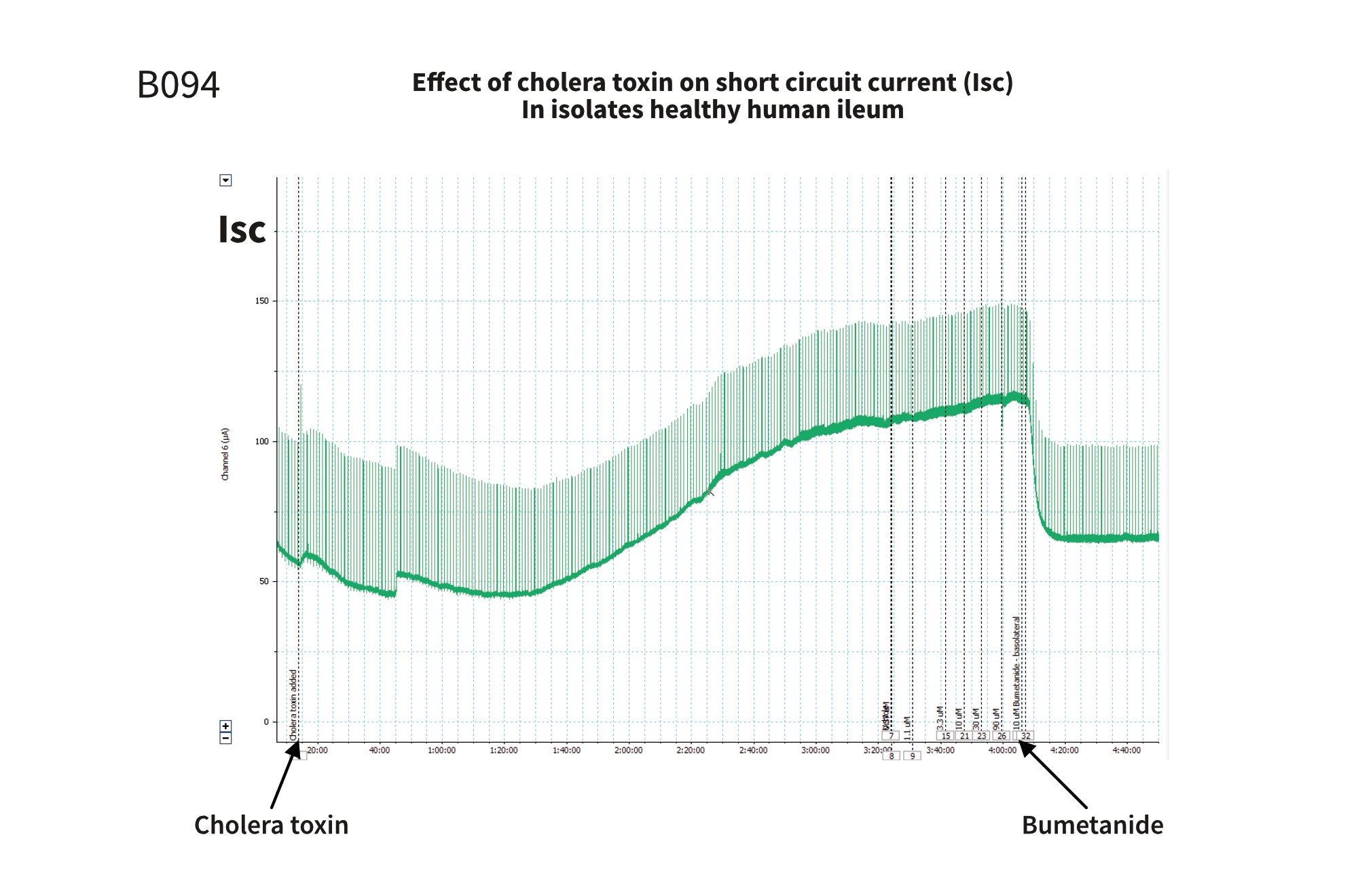 Figure 1: An increase in the short circuit current is observed following the addition of cholera toxin, corresponding to the increased efflux of ions to the apical side of the tissue. The addition of bumetanide causes a decrease in the short circuit current since bumetanide blocks the Na-K chloride transporter and inhibits the efflux.
Figure 1: An increase in the short circuit current is observed following the addition of cholera toxin, corresponding to the increased efflux of ions to the apical side of the tissue. The addition of bumetanide causes a decrease in the short circuit current since bumetanide blocks the Na-K chloride transporter and inhibits the efflux.
Testing Information
Introduction
The specific results that will be provided are the effects of increasing concentrations of test articles on the short circuit current across healthy human gastrointestinal mucosa.
Test Article Requirements
Test article(s) to be provided by the sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in deionised water unless otherwise requested. Bath volumes are 5mL; sponsor to provide sufficient test article to run the entire study.
Suggested Testing
In duplicate at 6 concentrations.
Study Outline
Rationale and Experimental Design
This assay assesses whether test articles cause a change in short circuit current across healthy human gastrointestinal mucosa. It is possible to assess ion channel function across multiple regions of the GI system.
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue. Furthermore, tissues which do not have a satisfactory potential difference or transepithelial resistance will be excluded.
Standardisation and Qualification
All GI mucosal tissues are initially processed through standardisation and qualification procedures to ensure functionality, prior to starting the study protocol.
Prior to mounting the mucosa in the Ussing Chambers, the potential difference of the electrodes and the resistance due to the PSS will be offset prior to the experiment. This will ensure that any electrical measurements obtained will be solely tissue derived.
Following mounting of the mucosa the tissue will be allowed to equilibrate before measurements of the tissue potential difference are made. If the tissue potential difference is satisfactory then the tissue will be placed under voltage clamp and the transepithelial resistance measured.
Tissue will be allowed to equilibrate under voltage clamp conditions before any experimental work is started.
Mucosal samples which pass the qualification pass/fail criteria will then progress to the study protocol.
Ion Channel Assays
Agonist Assays
To assess the ability of each test article to modulate ion channel function, 6 point cumulative concentration response curves will be performed for each test article. These concentration response curves (CCRC’s) will be performed either from baseline short circuit current or following addition of an appropriate agent. A positive control compound and representative test article vehicle CCRC will also be run to allow direct comparison with test articles.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate mucosal tissues):
-
Representative test article vehicle CCRC
-
Test article 1 CCRC
-
Test article 2 CCRC
-
Test article 3 CCRC
-
Positive control CCRC
To assess the involvement of a specific ion channel in any observed responses, a selective ion channel blocker can be added at the end of the test article CCRC.
Antagonist Assays
IC50 Determination
To assess the ability of each article to antagonise an agonist mediated modulation of ion channel function, 6 point cumulative concentration response curves will be performed for each test article. These concentration response curves (CCRC’s) will be performed following modulation of ion channel function with an appropriate reference agonist. A positive control compound and representative test article vehicle CCRC will also be run to allow direct comparison with test articles.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate mucosal tissues):
-
Representative test article vehicle CCRC
-
Test article 1 CCRC
-
Test article 2 CCRC
-
Test article 3 CCRC
-
Positive control CCRC
Analysis
Statistical analysis (where appropriate) will be performed using GraphPad Prism, with the results being shown in graphical form in the final report.