Alvetex Scaffold Application Note 12
Growth and Differentiation of Neural Tissues in Alvetex Scaffold
Download this application note as a PDF (14.2 MB)
Introduction
Understanding the cellular and molecular mechanisms that control the growth and differentiation of neural tissues is fundamental in the development of new therapeutic approaches to treat neurological disease and injury to the nervous system. In vitro cell-based assays play an important role in the modelling of cell behaviour in defined ways. Whilst existing in vitro approaches have been successful, there is increasing demand for enhanced systems with improved physiological relevance, to more closely mimic the functionality of cells in vivo.
This is especially relevant to models that study cell function in man, where human cells and tissues are used. Currently in neuroscience research, this is mostly limited to primary cadaveric tissue or established cell lines. Recent advances in stem cell research have developed methods to isolate and expand human stem cell populations the laboratory. Human stem cells represent a renewable source of progenitors that retain the capacity to differentiate into a range of alternative cell types, including human neural tissues. In this application note we demonstrate the application of two of REPROCELLʼs (formerly Reinnervateʼs*) technologies, the small molecule ec23, and the 3D cell culture technology, Alvetex™ Scaffold. We provide evidence to show that:
- Alvetex Scaffold technology can be used to grow and differentiate human pluripotent stem cells in 3D culture;
- the potent synthetic retinoid ec23 induces neural differentiation by stem cells in a robust manner;
- the production of neurospheres using ec23 to study neurite outgrowth;
- the combination of ec23-induced neurospheres with Alvetex Scaffold to produce a unique 3D neurite outgrowth assay to study neuritogenesis in a model mimicking the glial scar.
*Reinnervate Ltd was acquired by REPROCELL Inc in 2014. It was merged with Biopta Ltd to form REPROCELL Europe Ltd In 2016.
Highlights:
- Efficient induction of neural differentiation using a synthetic stable compound
- Robust production of neurospheres and enhanced formation of neurites
- Development of unique 3D neurite outgrowth assays
- Demonstration of glial / neuron co-culture model
Key Benefits:
- Reproducible and robust induction of neurogenesis by human stem cells
- Creation of more physiologically relevant models for neurobiologists
- Study cell-to-cell interactions in 3D co-cultures
- Improved models of pathological conditions in the damaged nervous system
Background
The regenerative capacity of human central nervous system (CNS) neurons is inhibited due to the formation post injury of the glial scar. Reactive astrocytes that migrate to the lesion site produce both inhibitory and permissive extracellular matrix (ECM) molecules. Inhibitory ECM molecules known as the chondroitin sulphate protoeglycans (CSPGs) are rapidly up-regulated by the reactive astrocytes after injury serving as a barrier which masks neurons from the neurite promoting components of the ECM. One such ECM is the CSPG known as Aggrecan which has been shown in vivo and in vitro to inhibit neurite regeneration [1]. It has been demonstrated in several models that Rho kinase (ROCK) inhibition can overcome CSPG inhibition of neurite regeneration. In addition the activation of retinoic acid receptor ꞵ2 (RAR ꞵ2) has also been shown to stimulate neurite outgrowth through cAMP up regulation and ROCK inhibition [2,3].
The small molecule ec23 developed by REPROCELL has previously been shown to enhance neural differentiation of both human neuroprogenitor and human embryonic stem cells [4]. The application of ec23 has been developed into a highly reproducible model to efficiently produce neurospheres that subsequently develop extensive neurites in conventional 2D culture vessels. Neurite outgrowth has been studied using this model and it was demonstrated that almost total neurite loss could be induced by the addition of 50 μg/ml aggrecan to a permissive laminin-coated substrate. Recovery of neurite growth by the addition of 10 μM synthetic ROCK inhibitor, Y-27632, was shown. Partial recovery was also demonstrated by selective activation of the retinoid RAR ꞵ2 receptor by the addition of synthetic compound AC261066 (10 μM).
This application note describes translation of this 2D system into a 3D model of neurite outgrowth using a combination of Alvetex Scaffold, ec23 and the human pluripotent stem cell line, TERA2.cl.SP12 [5]. In brief, addition of aggrecan to the scaffold induced an almost complete loss of 3D neurite outgrowth. Addition of both Y-27632 and AC261066 induced partial recovery of neurite outgrowth within the 3D model. A co-culture model was also developed using the glioblastoma cell line U118-MG to study the interaction between neurons and glia in 3D culture. These 3D models are reproducible and robust, and are more representative of the formation and function of the glial scar in vivo after CNS injury.
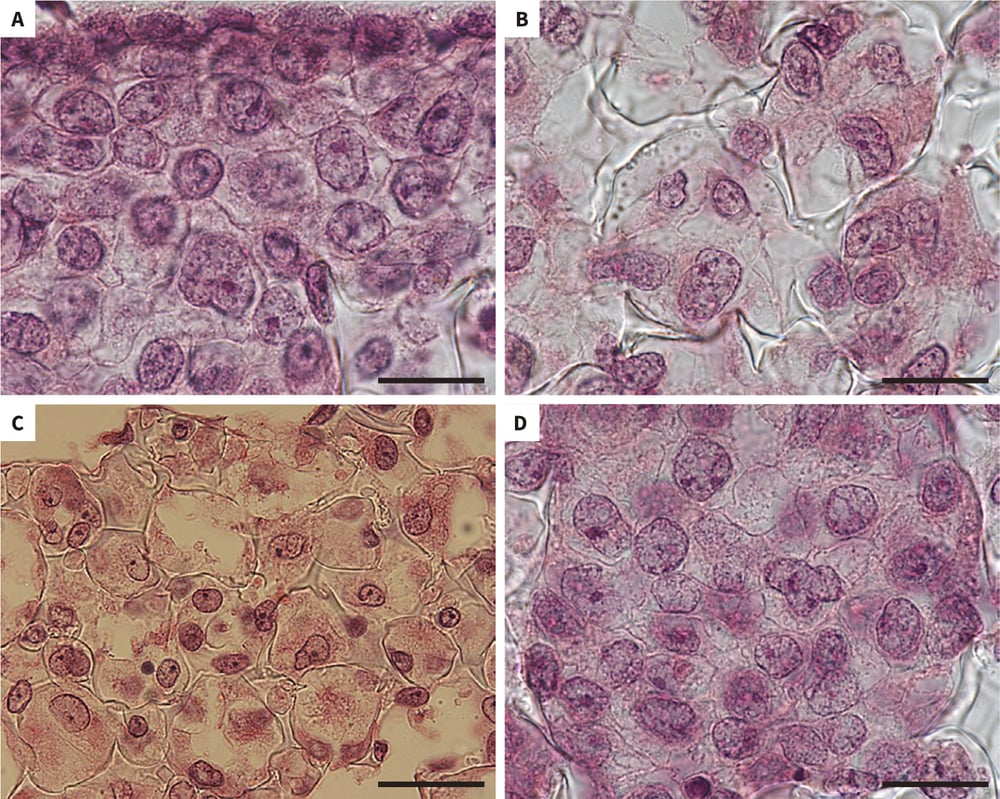
Figure 1. Growth of human pluripotent stem cells in Alvetex Scaffold. Brightfield micrographs of human pluripotent embryonal carcinoma stem cells (TERA2.cl.SP12) cultured in 3D using Alvetex Scaffold. Cells retain a natural rounded 3D structure and are closely packed together and possess a high cytoplasm to nucleus ratio. Scale bars: (A, B, D) 20 μm; (C) 40 μm.
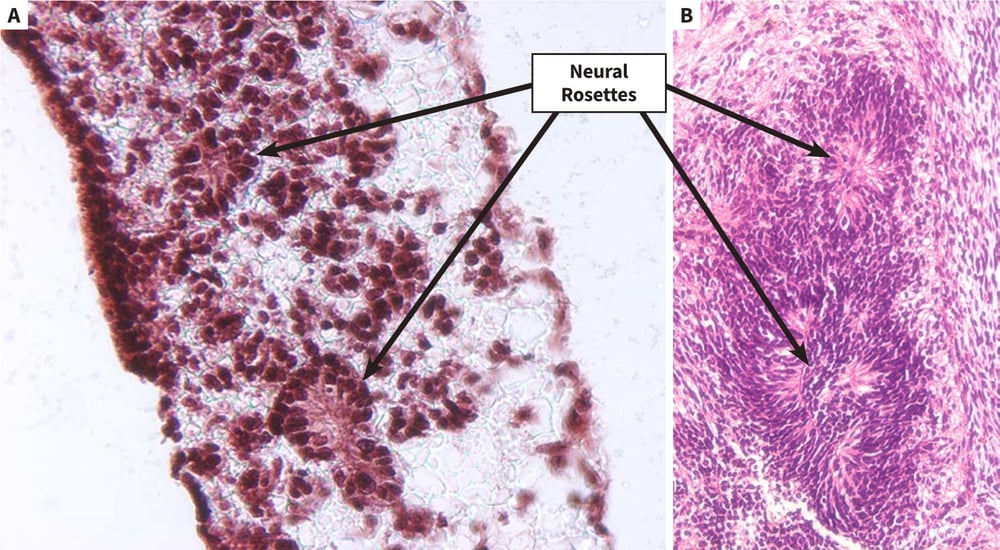
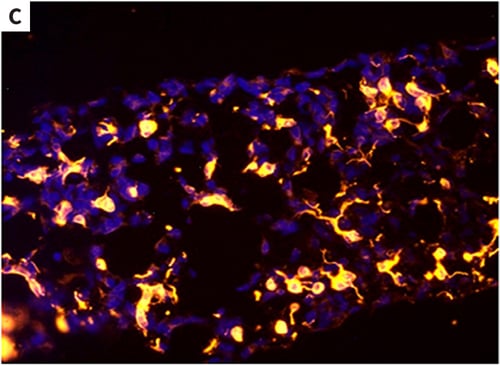
Figure 2. Differentiation of human pluripotent stem cells into neural derivatives in Alvetex Scaffold. Human pluripotent embryonal carcinoma stem cells (TERA2.cl. SP12) were grown in Alvetex Scaffold and exposed to retinoic acid (10 μM) for 14 days. Inside the scaffold, cells formed structures that resembled neural rosettes (A), as seen in teratomas derived from the same cells when transplanted into immune-deficient mice (B). Immunocytochemistry was used to confirm the identity of neural tissues by staining with TuJ1 antibody (C). Scale bars: (A) 40 μm; (B) 150 μm; (C) 65 μm.
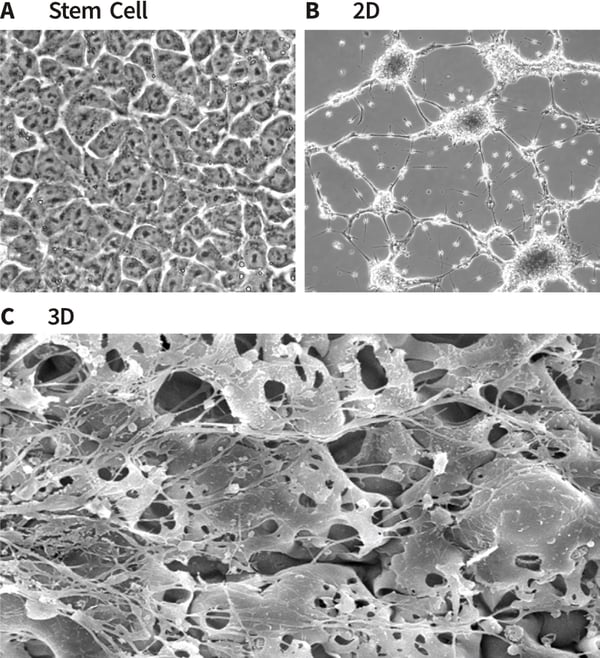
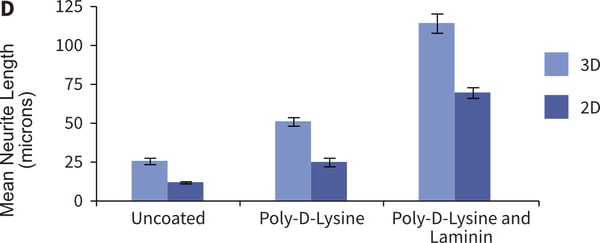
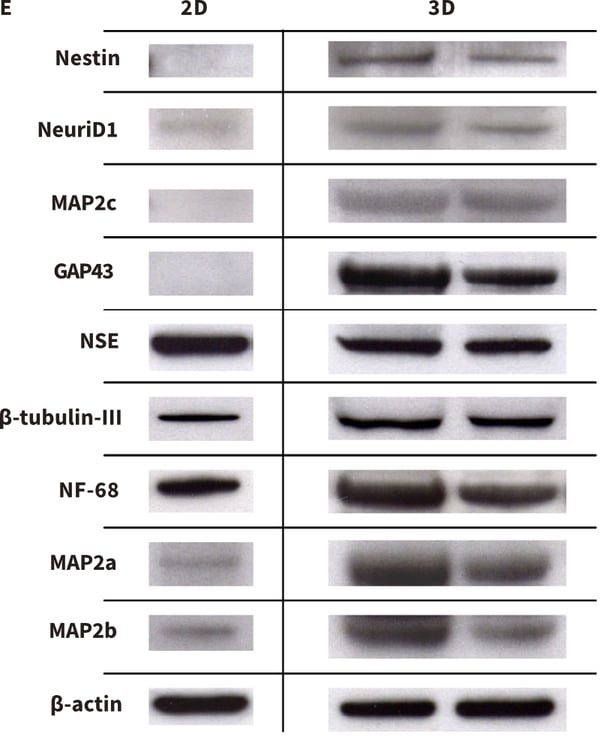
Figure 3. Formation of neurites by stem cell-derived neurons in 3D culture. Human pluripotent embryonal carcinoma stem cells (TERA2.cl.SP12, A) were differentiated into neurons and seeded either onto conventional 2D plastic-ware (B) or Alvetex Scaffold (C). Cells were imaged using either phase contrast microscopy (A, B) or scanning electron microscopy (C). Neurite length was measured and compared on 2D and 3D growth substrates with coatings of poly-D-lysine and laminin. The data show enhanced neurite length in cultures maintained in 3D culture (D). Protein lysed directly from cells in 2D or 3D culture was prepared for western blot analysis and a panel of neural markers assessed for protein (E). Markers of neurogenesis (e.g. nestin), neurite outgrowth (e.g. GAP43) and maturing neurites (e.g. MAP2b) were expressed at higher levels in cells maintained in 3D culture compared to 2D conditions. Collectively, these data suggest that neurite differentiation by stem cell-derived neurons in Alvetex Scaffold is significantly enhanced compared to their counterparts in conventional 2D cultures.
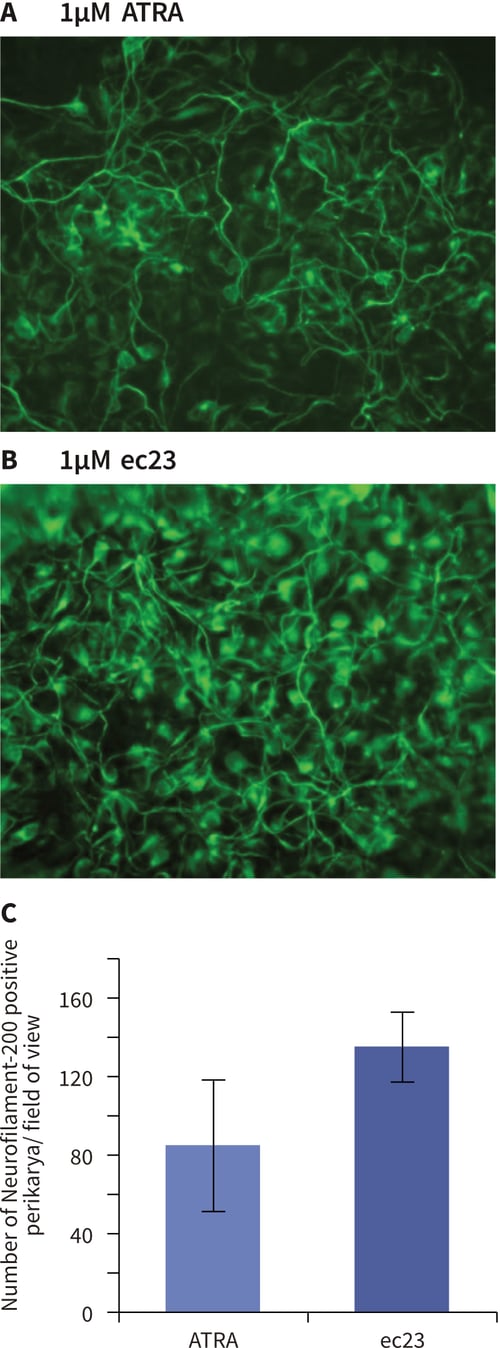
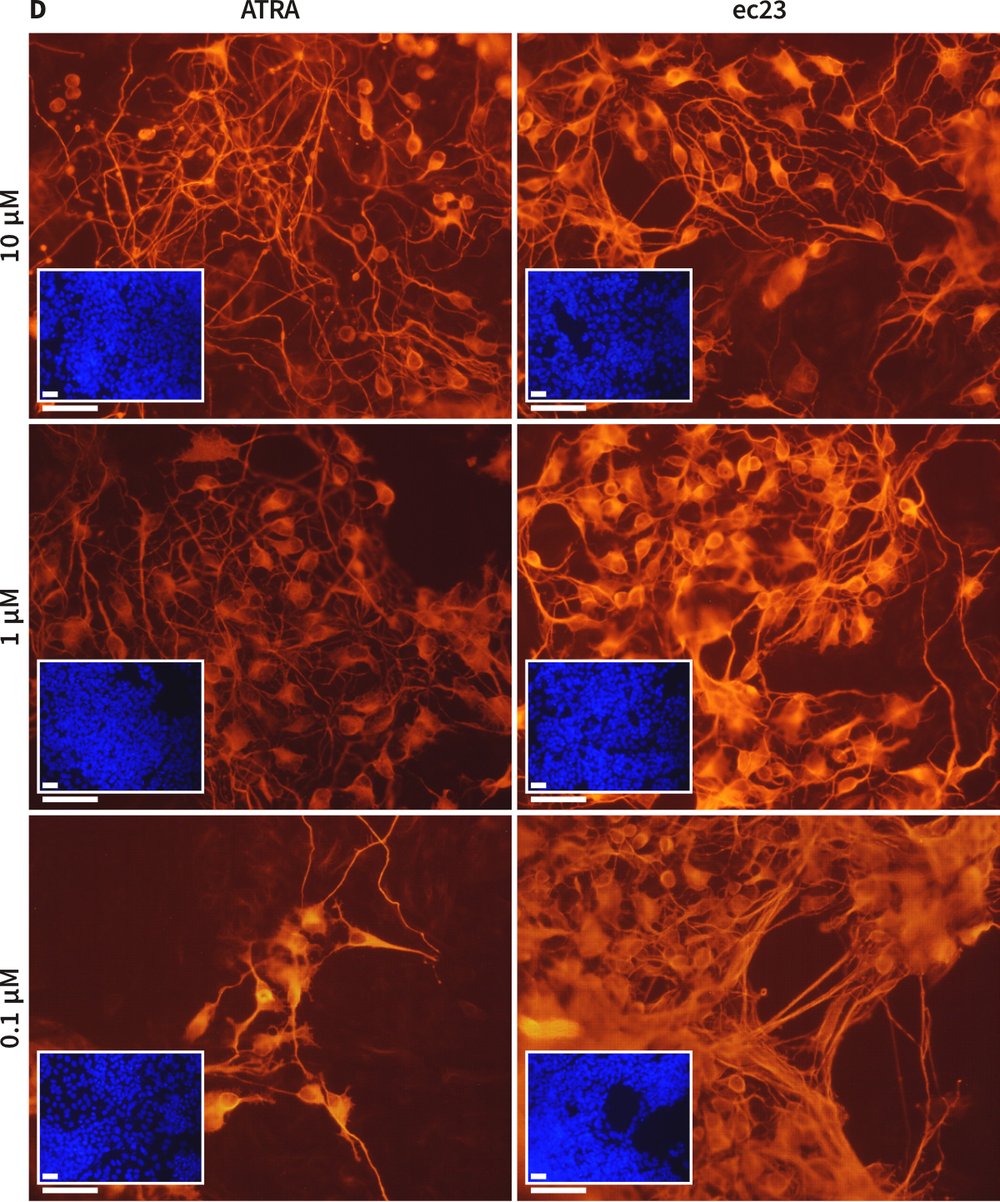
Figure 4. Enhanced induction of neural differentiation by stem cells in response to synthetic retinoic acid. Human pluripotent embryonal carcinoma stem cells (TERA2.cl.SP12) were exposed to either all-trans-retinoic acid (ATRA) or the synthetic retinoid, ec23 over a range of concentrations. Both retinoids induced the formation of neurons as identified by TuJ1 staining (A, B). In a direct comparison between the activity of 1 μM ATRA and 1 μM ec23, the number of neurons showing positive neurofilament-200 staining (a marker of the maturing axonal cytoskeleton) was significantly (40 %) greater in response to ec23 than ATRA (C, data represent mean ± SD, n=3). Concentration response experiments demonstrated clearly that ec23 retains a significant ability to induce neural differentiation down to a concentration of 0.1 μM whereas the response to ATRA was significantly reduced at this level (D, data show TuJ1 staining (red) of cells exposed to retinoids for 21 days, and a DAPI inset (blue) revealing the distribution of the total cell population in field of view, scale bars: 250 μm).
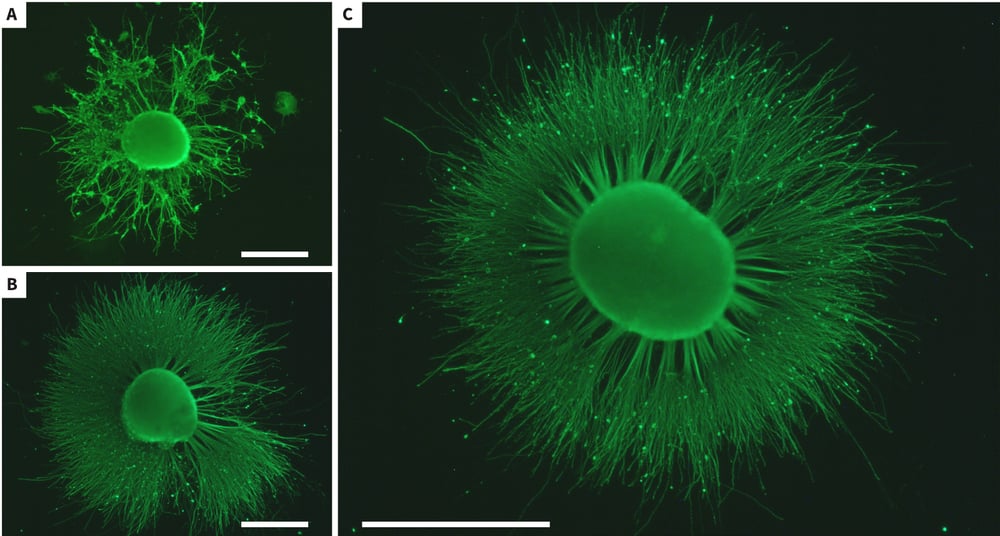
Figure 5. Enhanced neurite outgrowth from stem cell-derived neuroprogenitors in response to synthetic retinoic acid. Human pluripotent embryonal carcinoma stem cells (TERA2.cl.SP12) were differentiated in suspension cultures for 21 days in the presence of either ATRA (1 μM; A) or ec23 (1 μM; B, C). The resulting neurospheres were subsequently grown in media containing mitotic inhibitors and induced to form neurites on a 10 μg/ml laminin and poly-D-lysine substrate. Neurites were allowed to form for 10 days prior to fixation with 4% PFA and staining with TuJ1 antibody (green). Neurite outgrowth in response to ec23 (B) was significantly greater compared to that observed with ATRA (A). Panel (C) shows a typical example of extensive neuritogenesis observed from stem cell-derived neuroprogenitors exposed to ec23. Scale bars: (A-C) 500 μm.
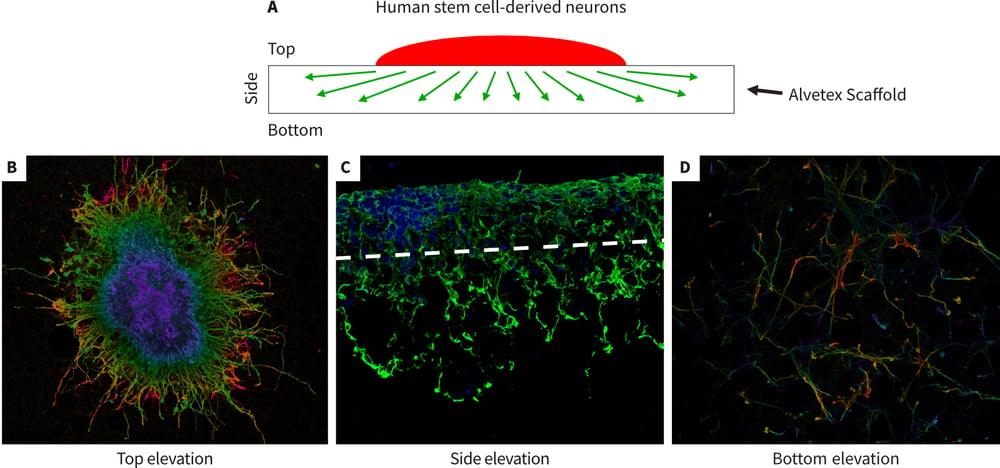
Figure 6. Development of a novel 3D neurite out-growth model using Alvetex Scaffold. Human pluripotent stem cell-derived neurospheres were seeded onto Alvetex Scaffold coated with 10 μg/ml laminin and poly-Dlysine and maintained for 10 days in media containing mitotic inhibitors. The neurospheres were produced from TERA2.cl.SP12 pluripotent stem cells exposed to 21 days ec23 as described above (Figure 5). After fixation with 4 % PFA, and staining with TuJ1, confocal microscopy was used to image the top, bottom and side elevations as illustrated in schematic diagram (A). Images were taken from samples that were either whole or wax embedded and sectioned preparations. Neurites grew out from the neurospheres, spreading laterally across and inside the scaffold (B) and also vertically, penetrating the depths of the material (C). In some cases, neurites had navigated across the full thickness (200 μm) of Alvetex Scaffold and were visible on the underside (D).
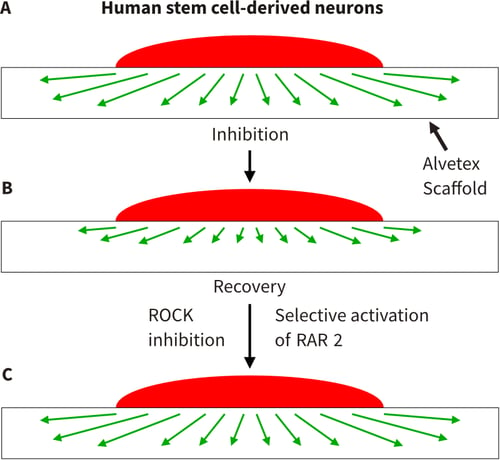
Figure 7. Developing 3D model to study inhibition of neurite outgrowth. A series of schematic diagrams illustrating the conceptual application of Alvetex Scaffold to create a 3D model to study neuritogenesis in response to known inhibitors of neurite outgrowth is shown. (A) 3D growth of neurites through Alvetex Scaffold as described above (Figure 6). (B) Inhibition of neurite outgrowth through coating Alvetex Scaffold with aggrecan, a chondroitin sulphate proteoglycan (CSPG). Aggrecan is found in the glial scar in the damaged spinal cord and has been demonstrated to inhibit neurite outgrowth and regeneration both in vitro and in vivo. (C) Methods to recover neurite inhibition are key to regeneration of the damaged spinal cord. Evidence in the literature suggests that manipulation of the ROCK and retinoid pathways enable partial recovery from CSPG inhibition of neuritogenesis. Inhibition of ROCK and selective activation of the retinoid RAR-ꞵ2 pathways can be achieved using the small molecules, Y-27632 and AC261066, respectively.
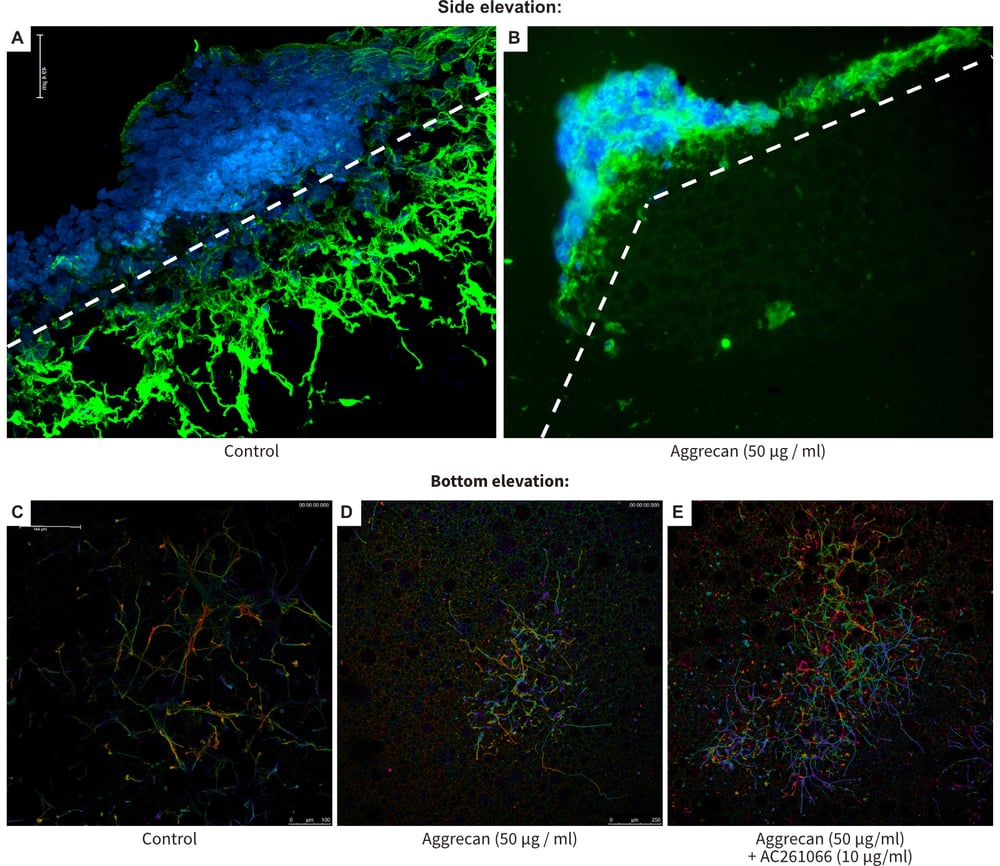
Figure 8. Recovery from neurite inhibition in a novel 3D model using Alvetex Scaffold. This Figure shows the outcome of a series of experiments that were designed to test the concept described in the schematics above (Figure 7). As in Figure 6, stem cell-derived neurospheres were produced and cultured on 10 μg/ml laminin and poly-Dlysine coated Alvetex Scaffold for 10 days prior to sectioning or bottom elevation confocal microscopy and staining with TuJ1 (green) and DAPI (blue) (A-E). Control conditions showed extensive neuritogenesis through the scaffold (A) and the presence of many neurites on the bottom of the material (C). Coating Alvetex Scaffold coated with 50 μg/ml of the chondroitin sulphate proteoglycan, aggrecan, suppressed neuritogenesis significantly (B) and far fewer neurites navigated the full thickness of the scaffold (D). Addition of 10 μM AC261066, a selective agonist of the RAR-ß2 retinoid receptor, resulted in greater numbers of neurites successfully traversing the scaffold in the presence of the inhibitory substrate (E).
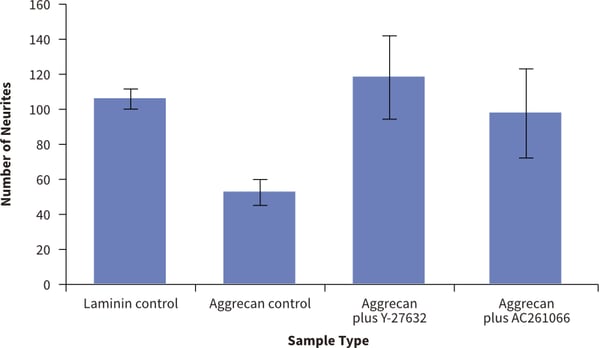
Figure 9. Recovery from neurite inhibition in a novel 3D model using Alvetex Scaffold. Quantification of the numbers of neurites observed on the bottom of Alvetex Scaffold was performed. This is evidence of neurites that have successfully navigated the full thickness of the 200 μm scaffold. Four different conditions were tested: (1.) positive control (laminin only); (2.) inhibitor control (laminin + aggrecan coating); (3.) ROCK rescue (laminin + aggrecan + Y- 27632); (4.) Retinoid rescue (laminin + aggrecan + AC261066). Counting the number of neurites that are present on the bottom of the alvetex after growing through the Alvetex Scaffold showed significant differences in the treatment groups (data show mean, ± SEM, n = 3 independent repeats). The values support the observations recorded above in Figure 8 and show rescue of 3D neurite outgrowth inhibition by small molecules known to promote neuritogenesis.
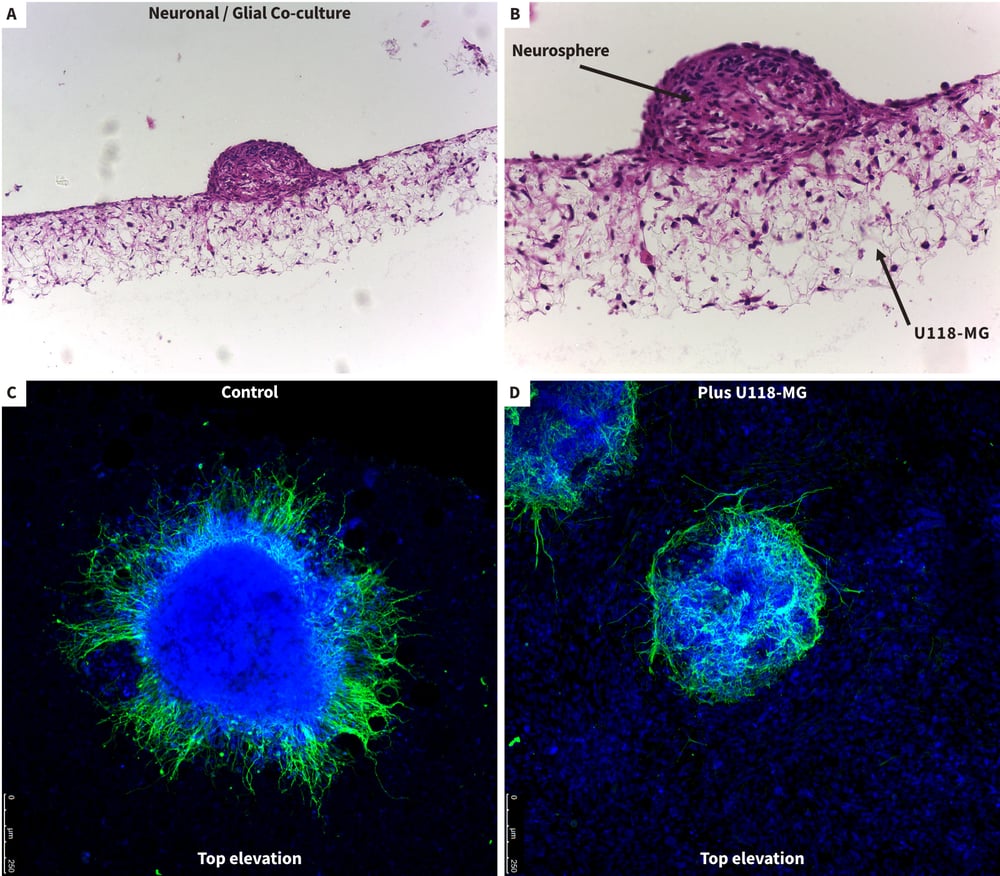
Figure 10. Studying interactions between neurons and glia in co-culture models using Alvetex Scaffold. Cocultures of glial cells (U118-MG) and stem cell-derived neurons were set up to study cellular interactions and the effect of glial cells on neuritogenesis. First, 1 million U118-MG glial cells were seeded onto 10 μg/ml laminin and poly-D-lysine coated Alvetex Scaffold and incubated for 15 min prior to the addition of neurospheres for a further 15 min. Co-cultures were incubated for 10 days and fixed in 4 % PFA prior to imaging as before. Bright field micrographs showing low (A) and high (B) magnification images of neurosphere and glial co-cultures on Alvetex Scaffold. Confocal imaging of neurospheres from above showed neurons producing neurites in the absence of U118-MG cells (C, control) and suppression of neuritogenesis in the presence of the glial cells (D, plus U118-MG). Cells were stained with TuJ1 (green) and DAPI (blue) to show neurons and cell nuclei, respectively. Note the uniform distribution of glia cells (D, DAPI stained nuclei) and how neurites tend to wrap around the neurosphere avoiding contact with the U118-MG cells (D).
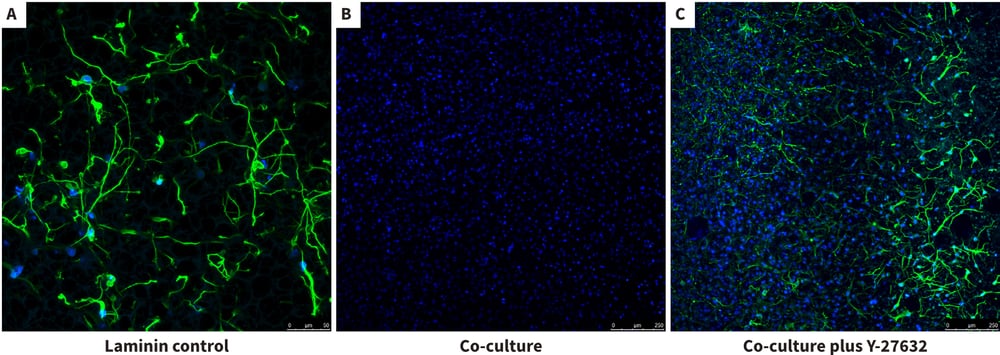
Figure 11. Inhibition and recovery of neurite outgrowth in neuron / glial 3D co-cultures using Alvetex Scaffold. In this example, we demonstrate further how the co-culture of U118-MG glia cells with the neurospheres suppressed neurite outgrowth but also how this effect can be rescued using the ROCK inhibitor Y-27632. Confocal microscopy of the bottom elevation in the neuron / glial co-culture model was performed as above to visualise neurites having navigated through the Alvetex Scaffold. Neurites were stained with TuJ-1 (green) and the glial cells were localised by DAPI staining (blue). Co-cultures were incubated for 10 days prior to imaging as before. The laminin control showed good evidence of extensive neurite outgrowth as before (A). In the presence of U118-MG cells, there was no evidence of neurites having successfully reached the bottom of the 3D culture (B). However, addition of the Y-27632 (15 μM) appeared to rescue the inhibitory influence of the glial cells and enable neurites to reach the bottom of the 3D co-culture (C).
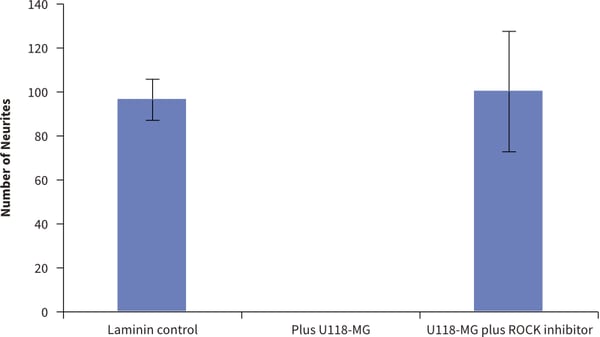
Figure 12. Inhibition and recovery of neurite outgrowth in neuron / glial 3D co-cultures using Alvetex Scaffold. Quantification of the numbers of neurites observed on the bottom of Alvetex Scaffold was performed as before but now in the presence of co-cultured U118-MG glial cells and neurons. Three different conditions were tested: (1.) positive control (neurospheres only); (2.) co-culture inhibition (U118-MG + neurospheres); (3.) co-culture rescue (U118-MG + neurospheres + Y-27632). Counting the number of neurites that are present on the bottom of the Alvetex Scaffold after growing through the scaffold showed significant differences in the treatment groups (data show mean, ±SEM, n=3 independent repeats). The values support the observations recorded above in Figure 11 and show that inhibition of neurite outgrowth by U118-MG glial cells can be overcome in the presence of the ROCK inhibitor, Y-27632.
References
- Tan et al. Integrin activation Promotes Axon Growth on Inhibitory Chondroitin Sulfate Proteoglycans by Enhancing Integrin Signaling. J Neurosci, April 27, 2011, 31(17):6289-6295.
- Lingor et al. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem, 2007, 103, 181-189.
- Jose Miguel Cosgaya and Ana Aranda. Nerve growth factor activates the RARꞵ2 promoter by a Ras-dependent mechanism. J Neurochem, 76, 661-670.
- Christie et al. Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org Biomol Chem, 2008,6, 3497-3507.
- Przyborski et al. Human embryonal carcinoma stem cells: models of embryonic development in humans. Stem Cells Dev, 2004 Aug; 13(4):400-8.
- Paquet-Durand et al. Turning teratocarcinoma cells into neurons: rapid differentiation of NT-2 cells in floating spheres. Dev Brain Res, 142, 2003, 161-167.