Histology (3): Processing Alvetex® Scaffold 3D Cultures for Bouin’s Fixation and Paraffin Wax Embedding for Haematoxylin and Eosin Staining
● Download this protocol as a PDF (2 MB)
1. Introduction
Following fixation a variety of different cytological stains can be used to visualise cell components in detail. Here we describe the use of Haematoxylin and Eosin, a general structural stain, one of the most commonly used stains throughout histology. The hemalum component of haematoxylin stains cell nuclei blue. Counterstaining of samples with an alcoholic eosin Y solution stains other eosinophilic structures in various shades of red, pink and orange. Most of the cell cytoplasm is eosinophilic and therefore stains pink. Alvetex Scaffold can easily be processed like a standard tissue sample, allowing established histology methods to be followed with excellent results. An example protocol is outlined below.
2. Materials and Methods
2.1. Materials Required
- Bouin’s fixative (for full recipe see Histology (1): Choosing the Right Fixative to Preserve 3D Cell Cultures)
- Dehydration ethanols (30 %, 50 %, 70 %, 80 %, 90 %, 95 %, 100 %)
- Histoclear
- Paraffin wax
- Mayers Haematoxylin solution
- Eosin solution (2 g eosin in 1000 ml 95 % alcohol)
- Alkaline alcohol
- DPX mountant
- Basic equipment: spade forceps to handle 3D cultures, scalpel (to trim/ cut Alvetex Scaffold culture if required), disposable pipettes, convection oven, embedding moulds, microtome.
2.2. Bouin’s Fixation of Scaffolds
- Aspirate off the medium and carefully wash the 3D culture in PBS twice.
- If using well inserts, remove inserts from cradle and place in a conventional 6-well plate. To fix, add 5 mL Bouin’s fixative at room temperature. Fix specimens at room temperature for a minimum of 12 hours but no longer than 36 hours.
- Aspirate off the fixative, wash three times using 5 mL PBS to thoroughly remove excess fixative, discarding the waste liquid.
- If using inserts, remove Alvetex Scaffold from the well insert either by separating the two parts of the device or using a scalpel to cut out the scaffold. At this time samples can be transferred to a tissue processing cassette to minimize direct handling and damage to the 3D culture.
- Aspirate off the PBS and add 5 mL of 30 % ethanol. Leave to equilibrate for at least 15 minutes. Aspirate off the ethanol and discard.
- Repeat with 50 %, 70 %, 80 %, 90 % and then 95 % ethanol. A gradual dehydration of the sample will result in less tissue shrinkage. Material can be stored in 95 % ethanol prior to paraffin embedding.
2.3. Paraffin Embedding and Sectioning
- Fully dehydrate specimens stored in 95 % ethanol by replacing with 100 % absolute ethanol for at least 30 minutes.
- Aspirate off the ethanol and replace with Histoclear for at least 30 minutes.
Note: Histoclear will dissolve certain types of plastic, therefore it is best to process samples in glass tissue processing cassettes.
- Replace the Histoclear with a 50:50 solution of Histoclear and molten paraffin wax (60 °C) mix and incubate in a convection oven at 60 °C for 30 minutes.
- Replace the Histoclear:wax mix with 100 % molten wax and incubate at 60 °C for a further 60 minutes.
- Transfer the scaffold to plastic embedding moulds and orientate into the required embedding position, with plane of section in mind. Embed in molten wax.
- Allow wax to cool and set at room temperature for 1-2 hours or overnight.
- Once the wax has hardened, remove the wax embedded block from the plastic mould. The sample is now ready for sectioning on a suitable microtome (e.g. Leica RM2125).
- Following the microtome manufacturer’s instructions throughout, align the block correctly with the microtome blade and proceed to cut 10 μm sections of the sample block.
- Transfer sections to a slide water bath (40 °C), floating them on the surface of the water to enable them to flatten out.
- Transfer selected sections to slides by flotation. Superfrost Plus slides (Thermo, 4951PLUS4) are recommended.
- Place on a slide drier and leave overnight. The sections should now be ready for staining.
2.4. Haematoxylin and Eosin staining
- Set up a series of slide chambers containing solutions as outlined in the following steps and process the slides using slide holders according to the indicated timings.
- Deparaffinise in Histoclear
- Transfer into absolute (100 %) alcohol
- Rehydrate through 95 % alcohol
- 70 % alcohol
- Distilled water
- Stain in Mayers Haematoxylin
- Wash in distilled water
- Blue the nuclei in alkaline alcohol
- Dehydrate in 70 % alcohol
- 95 % alcohol
- Stain in Eosin
- Dehydrate in 95 % alcohol
- 95 % alcohol
- Absolute alcohol
- Absolute alcohol
- Clear in Histoclear
- Histoclear
- Retain slides in Histoclear until ready to coverslip to prevent drying out.
2.5. Mounting and Cover-slipping
Mount in DPX by spotting DPX around the section and carefully cover with a coverslip ensuring no air bubbles are present. Allow to air dry for at least one hour.
3. Example results
Figure 1. shows an example of paraffin embedded 3D Alvetex Scaffold culture, sectioned and stained with Hematoxylin and Eosin.
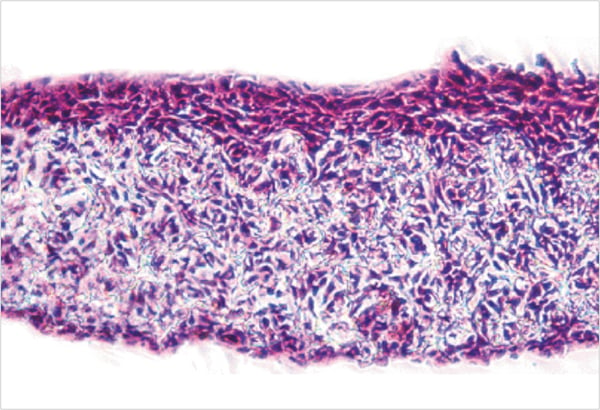
Figure 1. Human keratinocyte cell line (HaCaT) grown in Alvetex Scaffold (7 days air exposure). The subsequent culture was fixed in Bouins fixative and processed for paraffin wax embedding and sectioning. The sectioned materials (10 µm) were stained with Haematoxylin and Eosin for morphological analysis.