Inflamed skin model (PHA)
Drug Discovery Assay – reference number: B120
| Assay type: | Skin |
| Tissue: | Human skin biopsies (healthy) |
| Target: | Study dependent |
| Control compound: | Betamethasone |
| Study type: | Ex vivo cultures |
| Functional endpoint: | Inflammation |
Assay Description
This experiment assesses whether test articles cause a reduction in inflammatory cytokine release, with Betamethasone as a reference compound. It uses healthy skin punch biopsies which are induced to display an inflammatory phenotype using phytohemagglutinin (PHA). We then explore test article effect on the expression on inflammatory cytokines via ELISA and/or rtPCR.
Testing Information
Introduction
The specific results that will be provided are on the anti-inflammatory effect of test articles across an induced inflammatory model using human fresh skin.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in deionized water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
We suggest test conditions are assessed in triplet as a minimum.
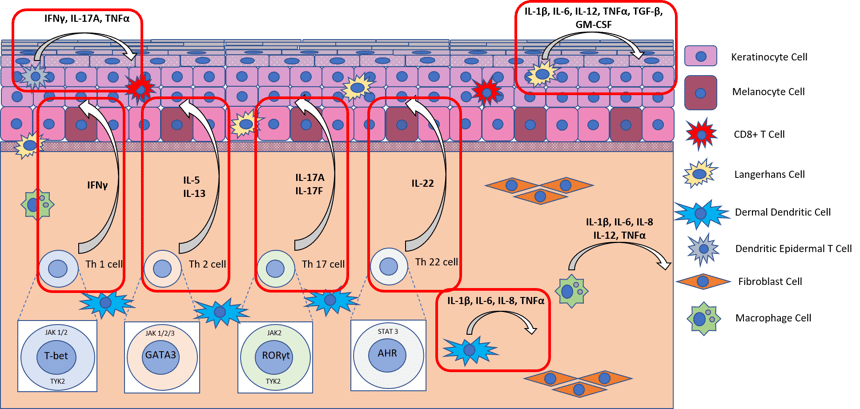
Diagram showing the molecular pathway activated in healthy skin by PHA
Study Outline
Rationale and Experimental Design
Full-thickness skin biopsies are achieved via punch-biopsy of residual surgical tissue. Biopsies are taken at a size of 3mm2 and transferred onto a mesh transwell in a 12 well plate. The dermis of the skin is submerged in specially fortified media, leaving the epidermis exposed to air. Biopsies are incubated in optimum conditions for a maximum of 24 hrs, then cultured in the presence of phytohemagglutinin and the test article for a further 24 hrs. Tissue media is collected for phenotype analysis by ELISA and/or the biopsy set is collected for rtPCR.
An example of the conditions assessed for 3 test articles are detailed below (it is recommended a minimum of triplicates should be used for each condition):
- Non-stimulated control
- Stimulated control
- Test article 1
- Test article 2
- Test article 3
- Positive control
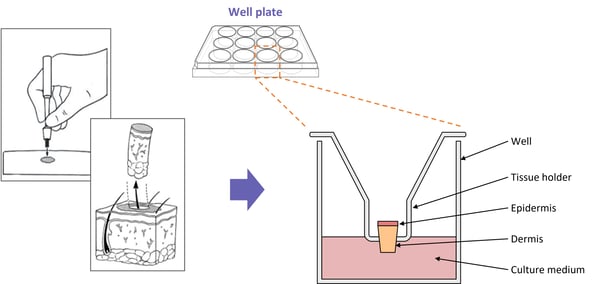
Diagram showing typical set-up for our inflamed skin model
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue.
Standardization and Qualification
Tissue that does not respond to stimulation with PHA would be deemed non-functional.
Analysis
Cytokine analysis: Supernatant samples can be analyzed for specific analytes of your choice by a multiplex ELISA platform. Each analyte will be quantified by interpolation against a standard curve generated on the same 96 well analysis plate.
Other forms of end point analysis are available such as gene expression or immunohistochemistry.
Example data from inflamed skin model
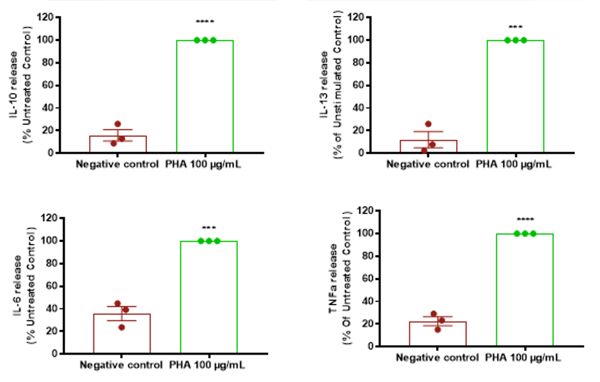
Induction of Inflammatory Cytokine Secretion after 24 h (systemic): Human skin explants stimulated with PHA showed a significant increase in the release of IL-6, IL-10, IL-13, TNFα and GM-CSF versus control skin (negative control). Data is presented as mean ± SEM in bar format with the individual donor means presented in the scatter. Analysis was conducted by unpaired two-tailed t-test. Three subjects were evaluated in triplicate measurements. Asterisks indicate significant (P < 0.05, for one, P < 0.01 for two, P < 0.001 for three and P < 0.0001 for four) differences versus the negative control group.
.jpg?width=756&height=425&name=Untitled%20design%20(10).jpg)

