Contractile Force in Electrically Stimulated Human Colon (Acetylcholine Esterase)
Drug Discovery Assay – reference number: B086
Overview
| Assay type: | GI tract |
| Tissue: | Human intestine (healthy) |
| Target: | Acetylcholine esterase |
| Control compound: | Galantamine |
| Study type: | EFS organ bath |
| Functional endpoint: | Contractile force |
Assay Description
This assay assesses whether test articles cause a change in contractile force on electrically stimulated human intestine, with galantamine as a reference compound.
The large intestine (colon) is essential for the absorption of fluid and electrolytes and the production of solid faeces. Changes in the anatomy or function of the large intestine can lead to disorders such as irritable bowel syndrome (IBS), diarrhoea and Ulcerative Colitis.
Testing Information
Introduction
The specific results that will be provided are the effects of increasing concentrations of test articles on the contractile state of isolated human intestines.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on diluent vehicle used. Stock solutions are prepared in distilled water unless otherwise requested. Bath volumes are 25mL; sponsor to provide sufficient test article to run the entire study.
Suggested Testing
In duplicate at 6 concentrations.
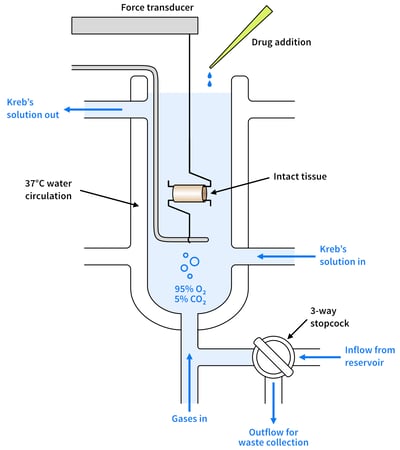 Figure 2: A diagram showing organ bath set-up
Figure 2: A diagram showing organ bath set-up
Study Outline
Rationale and Experimental Design
This assay assesses whether test articles cause a change in contractile force on electrically stimulated human stomach, with galantamine as a reference compound.
Exclusion Criteria
No specific exclusion criteria are in place other than to reject macroscopically diseased/necrotic tissue. Furthermore, tissues which do not respond to the standard pharmacology checks will be excluded.
Standardisation and Qualification
All individual muscle strips are initially processed through standardisation and qualification procedures to ensure functionality, prior to starting the study protocol.
Muscle strips are processed through a standardisation procedure to reduce signal variability prior to electrical field stimulation and pharmacological intervention. This ensures that muscle strips are maintained under appropriate physiological tension throughout the experiments.
Following standardisation, muscle strips are subjected to an electrical field stimulation (EFS) voltage curve. This process determines the optimal EFS settings to be utilised during the study protocol. Test tissues are then washed, and the EFS responses allowed to settle to steady baseline levels.
Muscle strips which pass the standardisation and qualification pass/fail criteria will then progress to the study protocol.
Smooth Muscle Contractility Assay
To assess their ability to modulate EFS mediated smooth muscle contractility, 6 point cumulative concentration response curves will be performed for each test article. These concentration response curves (CCRC’s) will be performed upon a steady baseline EFS response. A positive control compound and representative test article vehicle CCRC will also be run to allow direct comparison with test articles.
An example of the conditions assessed for 3 test articles are detailed below (each condition will be run in duplicate muscle strip preparations):
-
Representative test article vehicle CCRC
-
Positive control CCRC
-
Test article 1 CCRC
-
Test article 2 CCRC
-
Test article 3 CCRC
Analysis
Responses shall be expressed as % change from baseline EFS response. Statistical analysis (where appropriate) will be performed using GraphPad Prism, with the results being shown in graphical form in the final report.


