3D Bioengineered model of ageing human skin
Drug Discovery Assay – reference number: B129
Overview
| Tissue: | Bioengineered ageing full thickness skin equivalent |
| Target: | Study dependent |
| Control compound: | Application of test compounds |
| Study type: | 3D Cell Culture |
| Functional endpoint: | Transepidermal water loss (TEWL), histology, cytokine release, extracellular matrix (ECM) composition |
Testing Information
Assay Description
This platform can be used to assess the impact of test compounds on the structure and function of ageing skin, focusing on either epidermal or dermal factors. Changes can be monitored in comparison to untested controls and vehicle controls. The specific results that will be provided upon request are the effects of test articles on: TEWL, histology, cytokine release, ECM composition, ageing markers.
Test Article Requirements
Test article(s) to be provided by the Sponsor in storable aliquots at required test concentrations with information on the diluent vehicle used. Stock solutions are prepared in distilled water unless otherwise requested. Sponsor to provide sufficient test article to run the entire study.
Suggested Testing
A minimum of triplicate per condition with a maximum of 30 models per experiment. Several experiments can be run in a single study to increase model throughput.
Study Outline
Rationale and Experimental Design
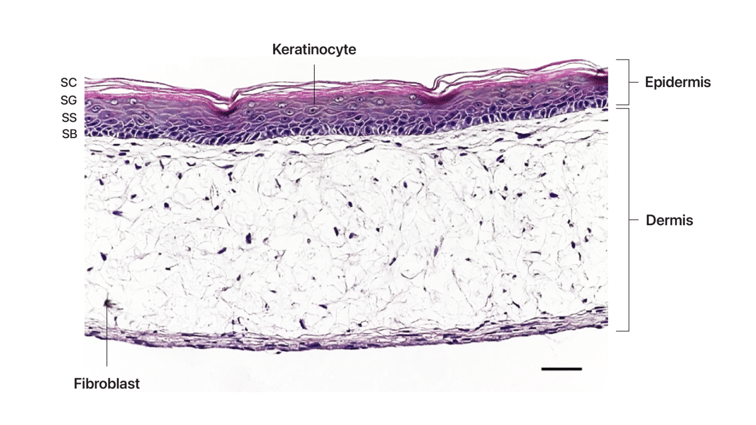
To construct the bioengineered ageing skin tissue, our researchers first generate a dermal foundation. The dermal foundation consists of fibroblasts seeded within Alvetex® Scaffold, which populate the scaffold and synthesise endogenous ECM, along with providing paracrine support to the overlying epidermal tissue in the form of soluble growth factors. The composition of the ageing dermal model may be customised dependent on the study requirements, for investigations into; chronological ageing, using fibroblasts from ageing donors; senescence, using neonatal fibroblasts of which a proportion have undergone senescence by chemical induction or exposure to high energy radiation, or a combination of the two approaches, in order to conduct a study on a complex ageing model. Keratinocytes are then seeded upon the dermal foundation and following proliferation and differentiation phases, form a mature construct at the air-liquid interface (ALI). Keratinocytes used match the identity of the major proportion of cells in the dermal compartment, either neonatal or ageing. Ageing donor cells may be available in a range of ages, dependent on the study requirements and supplier availability.

Schematic representation of culture process. Downstream applications include both topical and soluble formulation application or exposure to other exogenous stimuli. HDF – Human dermal fibroblasts, HEK – Human epidermal keratinocytes.
Once skin constructs have matured, application of the test substances can commence. This may be in the form of topical application, soluble addition to culture medium or exposure to other stimuli as desired. End point analyses include histology, inflammatory markers and ageing marker expression.
Addition of Test Articles
Test articles can take the form of solubilized compounds added directly to the culture medium, or topically applied formulations to the surface of the skin. It is recommended a minimum of triplicates should be used for each condition. An example of the conditions assessed for three test agents would be as follows:
- Untouched Control
- Vehicle Control
- Test Agent 1
- Test Agent 2
- Test Agent 3
End Point Analysis
There are a range of endpoints that can be used to assess effectiveness in this system including:
- Barrier Integrity: Histological assessment, immunofluorescent staining, TEWL measurements
- Dermal Changes: immunofluorescent detection of ECM components, quantitative ECM assays
- Ageing associated changes: Histological assessment, cytokine secretion, immunofluorescent detection of ageing markers
Example data
Ageing model structure: Models created using diverse ageing populations display a distinct phenotype and secrete inflammatory cytokines associated with ageing
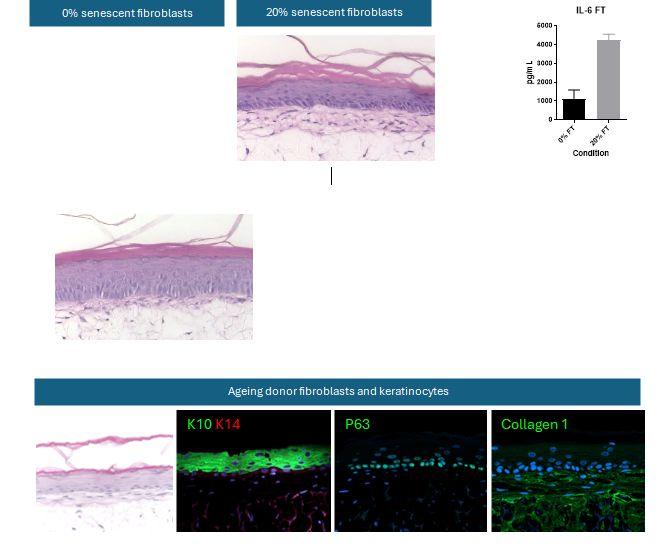
Models with increasing senescent cells display epidermal thinning and alterations in tissue morphology, as well as secreting proinflammatory cytokines known to be involved in the senescence associated secretory phenotype (SASP), such as IL-6. Models comprised entirely of ageing cells displays the hallmarks of in vitro skin models (localised differentiation markers, proliferation markers and ECM deposition) but with a distinct phenotype; reduced polarisation of basal cells, a thinner epidermis and impaired differentiation. (Data represent mean ± SEM, n = 3 experiments).
ECM deposition: Incorporation of senescent cells into the dermal compartment reduces total collagen content of the ECM

Incorporation of increasing fractions of senescent fibroblasts into a neonatal dermal compartment results in the reduction total collagen content compared to control models with no senescent fibroblasts. Assessment of collagen content can be used as an endpoint due to general alterations in ECM components observed in ageing skin. Dermal models were assessed at 28 days. (Data represent mean ± SEM, n ≥ 3 models). Statistical significance described compared with control, *** indicates p<0.001, ** indicates p<0.01, One way ANOVA.
References
Low E, Smith LA, Miwa S, Fielder E, Przyborski S, von Zglinicki T, Senescent dermal fibroblasts decrease stemness in basal keratinocytes in a bioengineered model of human full thickness skin, The Journal of Investigative Dermatology (2024), doi: https://doi.org/10.1016/ j.jid.2024.07.004.