This blog is the first in a series exploring the critical role of safety pharmacology in drug discovery. In this post, we delve into the importance of human tissue studies in ensuring drug safety. Stay tuned for our next installment, where we will showcase real-world case studies highlighting our innovative approaches and successes in using human tissue models for safety assessment.
In the complex journey of drug discovery, one of the most critical phases is ensuring that a potential therapeutic is not only effective but also safe for human use. Safety pharmacology plays a pivotal role in this process, especially when it involves studying human tissues. By understanding the impact of a drug on various human tissues, researchers can predict and mitigate potential adverse effects, ensuring that only the safest drugs proceed to clinical trials.
What is Safety Pharmacology?
Safety pharmacology evaluates the possible risks that new pharmaceuticals may pose to human health.1 It also evaluates potential adverse effects of new chemical entities (NCEs) on physiological functions. It’s a key component of preclinical research that aims to anticipate and mitigate any risks associated with the use of a new drug.
The focus of safety pharmacology studies is typically on vital organ systems, including the cardiovascular, respiratory, and central nervous systems. However, as science progresses, there is an increasing emphasis on understanding drug effects on other tissues, particularly human tissues, to enhance the predictive value of these studies.
The Importance of Human Tissue Studies
Human tissue studies have gained prominence in safety pharmacology due to their ability to provide more accurate and relevant data compared to animal models. While animal studies are invaluable, they do not always fully predict human responses. Differences in physiology, metabolism, and genetic makeup between species can lead to discrepancies in how a drug behaves in animals versus humans.
Human tissue studies allow researchers to observe how a drug interacts with specific human cells, tissues, or organs. This can be done using ex vivo tissue samples, primary cell cultures, or advanced models like organ-on-a-chip technologies. These studies help in identifying potential off-target effects, toxicities, and other adverse outcomes that might not be evident in animal studies.
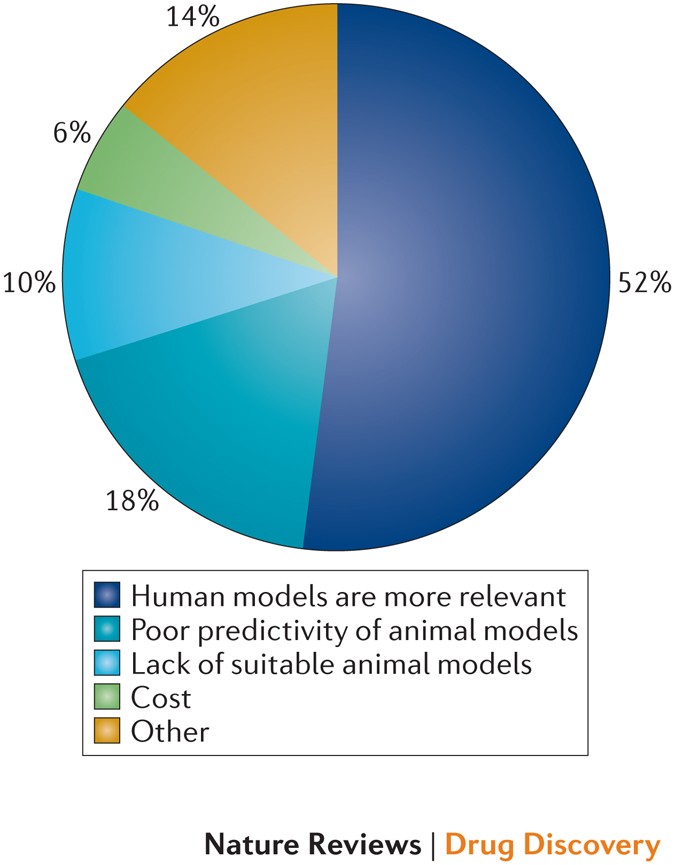
The figure above shows the reasons for adopting human tissue-based methods in safety pharmacology studies, as taken from Nature, "Assessing drug safety in human tissues — what are the barriers?" - cited below.2
Key Benefits of Safety Pharmacology in Human Tissues
- Improved Predictability: By using human tissues, researchers can obtain data that is more directly translatable to human clinical outcomes. This reduces the likelihood of unexpected adverse effects when the drug is administered to humans.
- Reduction in Animal Use: Incorporating human tissues in safety pharmacology aligns with the ethical imperative to reduce, refine, and replace animal testing where possible. This not only addresses ethical concerns but also enhances the scientific relevance of the data.
- Early Detection of Adverse Effects: Human tissue studies allow for the early identification of potential toxicities or adverse effects, providing a critical checkpoint before proceeding to more costly and time-consuming clinical trials.
- Customization for Specific Populations: Human tissue studies can be tailored to reflect specific patient populations, such as those with genetic predispositions or existing comorbidities, offering insights into how these factors may influence drug safety.
Challenges and Future Directions
Despite the advantages, there are challenges in implementing human tissue studies in safety pharmacology. These include the limited availability of high-quality human tissues, the complexity of replicating the in vivo environment in vitro, and the need for standardized protocols and regulatory acceptance.
However, advancements in technology are helping to overcome these hurdles. Innovations such as induced pluripotent stem cells (iPSCs), 3D tissue cultures, and organoids are expanding the possibilities for human tissue studies. Additionally, regulatory bodies are increasingly recognizing the value of these models in enhancing drug safety, paving the way for broader acceptance and integration into the drug discovery pipeline.
Growing Demand for Human Tissue Models
Recent data published by Nature Reviews Drug Discovery highlights a significant and growing interest in human tissue-based approaches within the drug discovery community. According to the study, 63% of respondents are aware of human tissue-based approaches currently under evaluation within their organizations, and a remarkable 93% intend to use these assays in the future. This trend underscores the increasing appetite for developing and utilizing human tissue models in safety assessment, reflecting a broader shift in the industry towards more human-relevant methodologies.
The demand for human tissue models spans both core and non-core battery studies within safety pharmacology, as well as toxicology. Perhaps unsurprisingly, cardiovascular safety assessment shows the greatest demand for human models, with 56% of respondents indicating a need in this area. This demand is fairly consistent across other safety pharmacology endpoints and toxicology, highlighting the growing recognition of the value of human tissue models in a comprehensive safety assessment strategy.2
Conclusion
Safety pharmacology in human tissues represents a crucial step forward in the drug discovery process. By providing more accurate, relevant, and ethical data, these studies help ensure that new therapeutics are both effective and safe for human use. As the field continues to evolve, integrating human tissue studies will become increasingly essential, contributing to the development of safer drugs and ultimately improving patient outcomes.
For drug discovery companies, investing in human tissue-based safety pharmacology is not just a scientific imperative but a strategic one. It positions companies at the forefront of innovation, ensuring that they can deliver safe, effective, and high-quality therapeutics to the market, while also aligning with evolving regulatory standards and ethical considerations.
References
1. Porsolt R. Overview of Safety Pharmacology, Current Protocols in Pharmacology (2006).
2. Holmes A et al. Assessing drug safety in human tissues - what are the barriers? Nature Reviews Drug Discovery 14, pages 585–587 (2015).



.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



