Three-dimensional (3D) in vitro culture can improve the performance and physiological relevance of immortalized and primary cells. However, it increases proliferation rates in some cell types (e.g. mesenchymal stem cells, osteosarcoma cells, HUVECs) which puts extra demands on nutrient supply and waste removal [1,2,3]. This can be addressed by the use of a perfusion system that circulates a large quantity of media over or around the 3D culture. The systems have been shown to improve cell proliferation and specific protein expression beyond that seen for static 3D cultures [4,5,6,7].
In this post, we will explore two examples of how perfusion systems can help improve the relevance of your 3D cell culture, based on two publications from Alvetex users in the field of developmental biology and cancer.
Alvetex Perfusion Cell Culture System improves conditioned medium activity in paracrine co-cultures
In a 2017 Bone Reports study8, researchers from the University of Melbourne and from Boston University teamed up to investigate the crosstalk between bone and muscle; more specifically, the effect of osteocyte-conditioned medium on myogenic differentiation. MLO-Y4 osteocytes were cultured in Alvetex scaffold in either perfused or static conditions (using a perfusion plate or well inserts respectively) and the resulting conditioned medium was added to C2C12 myoblasts.
Several cytokines relevant to muscle formation were detected in the conditioned medium, but the volume of individual cytokines varied between the static and perfused medium. There was a decrease in IL-1β, IL-3, IL-4, IL-10, MIG, and MIP-1a in the perfused culture conditioned medium compared to its static culture equivalent suggesting a greater inhibitory activity of the perfused conditioned medium.
A
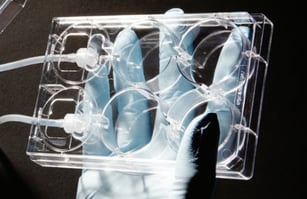
B
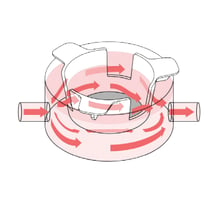
Figure 1: A, Alvetex Perfusion Culture Plate uses a modified 6-well plate design well-known to lab scientists. B, Pairing the Alvetex Perfusion Culture Plate with Alvetex well inserts combines the advantages of medium flow with 3D cell culture.
Alvetex Perfusion Cell Culture System maintains metabolic homeostasis during Cancer cell culture
In a separate project, scientists from the University of Oxford cultured the human pancreatic cancer cell line PSN-1 over a period of seven days, in either static or perfused conditions, using the Alvetex perfusion plate. The levels of glucose and secreted lactate contained in the cell culture medium were monitored as indicators of metabolic depletion, while protein syntheses and cell proliferation were measured by expression of phosphorylated Ribosomal protein S6 and cyclin D1. Results suggested that perfusion culture reduces hypoglycemia, anaerobic stress, and preserves protein synthesis and cell proliferation.
A
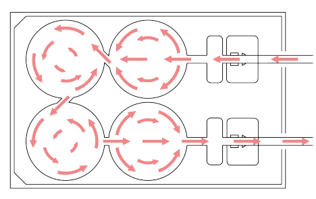
B
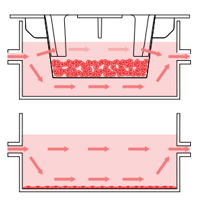
Figure 2: A, Unidirectional flow through 4 interconnected wells within each Alvetex Perfusion plate. B, Each well can support 2D or 3D culture, for a range of co-culture options.
Meet the inventor of Alvetex: Video Interview with Prof Stefan Przyborski
Adding Perfusion to your 3D Cell Culture the Easy Way
If this is the kind of results you want to achieve with your in vitro cell models, then adding perfusion can be done with minimal disruption to your existing protocols. Alvetex Perfusion plates:
- Follow a modified 6-well plate design, so they are compatible not only with our Alvetex well inserts, but with most commercial 6-well inserts.
- Are fitted with standard luer locks, so they can be connected to whichever model of tubing and pump are most adapted to each user’s applications.
- Come with no previous cell-compatible coating, so they can be used for non-adherent culture or be coated with each user’s preferred ECM.
- Have 4 connected wells, allowing for greater volume provision for highly metabolic monocultures, or active solute flow for paracrine or cell migration co-cultures.
Could perfusion reduce metabolic stress in your cultures and improve the functionality of your cells? Get in touch with one of our scientists to find out. We’re looking forward to learning more about your work and how our systems could help you take your research further.
References
- Chang et al. In vitro expansion of mesenchymal stem cells using 3-D matrix derived from cardiac fibroblast. Tissue Engineering and Regenerative Medicine 4:3 (2007)
- Chen et al. Effects of three-dimensional culturing on osteosarcoma cells grown in a fibrous matrix: analyses of cell morphology, cell cycle, and apoptosis. Biotechnology Progress 19:5 (2003)
- Chopra et al. Three-dimensional endothelial-tumor epithelial cell interactions in human cervical cancers. In Vitro Cellular & Developmental Biology 33:6 (1997)
- Pu et al. The use of flow perfusion culture and subcutaneous implantation with fibroblast-seeded PLLA-collagen 3D scaffolds for abdominal wall repair. Biomaterials 31:15 (2010)
- Dvir et al. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Engineering 10:12 (2006)
- Gomes et al. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Engineering 12:4 (2006)
- Oliver et al. In vitro culture of large bone substitutes in a new bioreactor: importance of the flow direction. Biomedical Materials 2:3 (2007)
- Wood et al. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Reports 6: (2017)
Editor's note: This blog was first published in 2019 but has since been updated for accuracy and clarity.


.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



