There are many benefits to the introduction of iPSC-derived therapies including improved patient quality of life, decreased clinical drug attrition rates, and reduced pressure on healthcare systems. However, there is still much work to be done before these therapies can become clinically available. In this post, we will explore three challenges currently facing the widespread introduction of iPSC therapies to regenerative medicine.
What is regenerative medicine?
Regenerative medicine is an interdisciplinary field that applies engineering and life science principles to heal or replace tissues and organs damaged by age, disease, or trauma. Some examples of regenerative therapies include universal blood donation, cellular therapies, and whole organ replacement. You can read more about the future applications of stem cell therapies in regenerative medicine here.
1. iPSC Reprogramming safety
Over 40% of all REPROCELL customers are concerned about the safety of iPSC therapeutics – and they are not alone. One key issue is the risk of mutation during reprogramming which can lead to tumor formation and reduced differentiation capacity. These genetic alterations – including chromosomal aberrations, copy number variations (CNVs), and single nucleotide variations (SNVs) – currently occur in 38-48% of iPSCs generated using integrative reprogramming methods.
The risk of tumor formation can be reduced by employing an integration-free reprogramming methodology. These non-integrative reprogramming methods include StemRNA™ 3rd Gen Reprogramming Technology. This RNA Reprogramming methodology carries the highest reprogramming efficiency and produces iPSCs of superior viability, pluripotency, and quality compared with other non-integrative reprogramming methods.
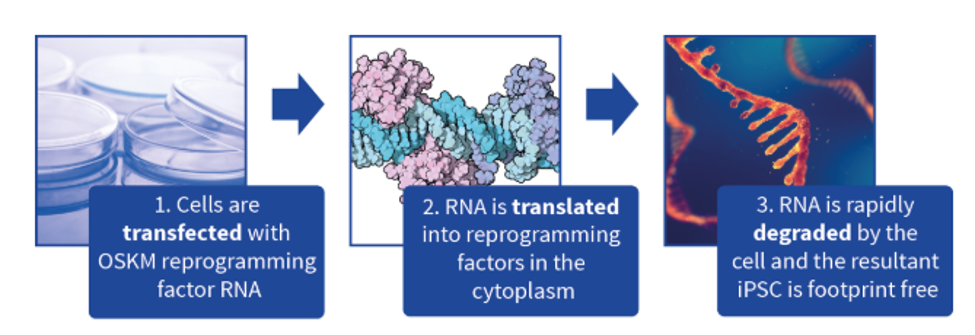
Figure 1. StemRNA™ 3rd Gen Reprogramming Technology. RNA Reprogramming is an efficient, footprint-free method for restoring pluripotency in adult cells.
2. Lack of automated production tools
If iPSC therapeutics are to become mainstream, this will require the development of more automated production tools. Already, bioreactor culture systems have been introduced for the rapid expansion of pluripotent stem cells, such as those provided by ABLE Biott and TreeFrog Therapeutics.
However, many more systems for the automation of stem cell reprogramming, differentiation, and analysis require development before the widescale introduction of these therapeutics will be possible. The video below shows how these semi-automated bioreactor systems allow the mass culture of induced pluripotent stem cells.
3. Difficulty in iPSC gene editing
Stem cells are notorious for their gene-editing difficulty, as it is challenging to maintain their pluripotency throughout the rounds of experimental optimization. Substantial experience in both molecular and stem cell biology is necessary to genetically modify iPSCs, and there are few companies or individuals skilled in both areas.
REPROCELL has developed an AI-enabled gene editing technology, empowering gene modifications with low off-target effects. Synthego[1] has also recently expanded its CRISPR gene-editing portfolio to include iPSCs, including SNP mutations, knock-out modifications, and tagged lines to make this process easier for those developing stem cell therapeutics.
References
- Synthego webpage
- Kim HO. In vitro stem cell-derived red blood cells for transfusion: Are we there yet? Yonsei Medical Journal 55:2 (2014)
- Douay L. Why industrial production of red blood cells from stem cells is essential for tomorrow’s blood transfusion. Regenerative Medicine 13:6 (2018)
- Mao et al. Regenerative Medicine: Current therapies and future directions. PNAS 112:47 (2015)
- Turinetto et al. Induced Pluripotent Stem Cells: Advances in the Quest for Genetic Stability during Reprogramming Process. International Journal of Molecular Sciences 18:9 (2017)
- Kang et al. Effects of Integrating and Non-Integrating Reprogramming Methods on Copy Number Variation and Genomic Stability of Human Induced Pluripotent Stem Cells. PLos One 10:7 (2015)
- Haridhasapavalan et al. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 20:686 (2019)
- Schlaeger et al. A comparison of non-integrating reprogramming methods. Nature Biotechnology 33:1 (2015)
Note: The modified cells are developed, manufactured or supplied by GenAhead Bio Inc. under license from ERS Genomics Limited.


.jpg?width=1000&height=562&name=Untitled%20design%20(10).jpg)



